Large-volume cryopreservation
The controlled cooling of small sample volumes is common in many scientific cryopreservation applications. The ability to cryopreserve larger volumes, for example in the development and production of many late-phase gene and cell therapies, is becoming increasingly desirable as more licensed treatments become available.
CAR T cell therapies, as well as blood products and cord blood, are some common examples of where large-volume cryopreservation is common. This is due to the large cell numbers required for effective treatment, as well as processing limitations. In all applications, it is critical that cell viability and function are maintained during the cryogenic processing and after thawing.
In many cases, liquid nitrogen (LN2) is the default choice for cryopreservation, pumped into the cooling chamber directly. Use of LN2 can give rise to concerns of contamination and sterility, safety, and additional running costs. Samples nearest to the LN2 source will also cool at a different rate than those furthest away, leading to inconsistent cooling and concerns regarding cell viability.
This study demonstrates a cell freezing protocol that uses the LN2-free VIA Freeze Quad controlled-rate freezer for the cryopreservation of up to eight cryobags and up to 560 mL total volume. Similar protocols can be applied to the VIA Freeze and VIA Freeze Duo controlled-rate freezers for cryopreservation of up to 140 mL and 280 mL, respectively.
Using this VIA Freeze protocol, thermal profiles and biological outcome in terms of post-thaw viability are equal between different bags in a “rack” set-up and unaffected by the orientation of the cryobags (vertical or horizontal). Also, the same cooling rates and biological results are achieved whether cryopreserving only one bag, or eight, indicating that the proportion of loading capacity used does not impact VIA Freeze performance.
Methods for freezing multiple cryobags
Two VIA Freeze multi-cryobag plates (Cytiva) were placed into a VIA Freeze Quad controlled rate freezer (Cytiva) with a VIA Freeze Quad 80 mm lid spacer (Cytiva). This set-up enables the cryopreservation of up to eight cryobags with a fill volume of 70 mL each (Fig 1).
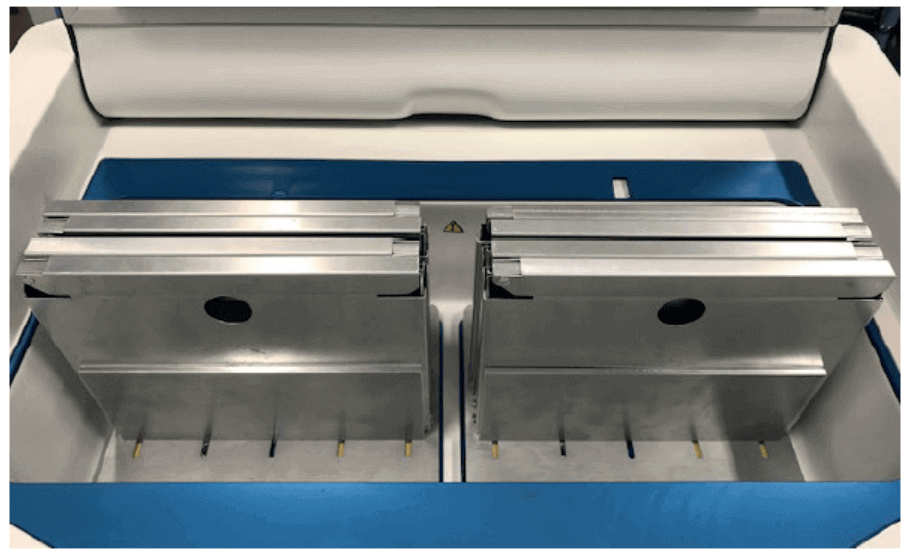
Fig 1. A view of the cryochamber within the VIA Freeze Quad unit with sample plates loaded with 8 × 70 mL cryobags held in metal cassettes.
Eight CryoMACS™ 250 bags (Miltenyi Biotech) were each filled with 70 mL of Jurkat cell suspension at a density of 3 × 106 cells/mL. The cryoprotective medium suspending the cells consisted of 5% DMSO in RPMI-1640 (Gibco), supplemented with 10% HyClone Calf Serum, U.S. origin (Cytiva).
The cells were cooled using the protocol shown in Table 1 before being transferred to ultra-low temperature storage at -140°C (transfer to long-term storage is possible once temperatures fall below -50°C). As nucleation is a stochastic event, a hold step is used during cooling to allow ice formation and temperature equilibration.
| Cryopreservation step | Purpose |
|---|---|
| Room temperature to 4°C at 1°C/min | At 4°C, samples were loaded to the VIA Freeze, to reduce DMSO toxicity |
| 4°C to -35°C at 0.75°C/min | Initial cooling and freezing of sample |
| 30 minute hold at -35°C | A hold to allow the whole sample to freeze after ice nucleation |
| -35°C to -60°C at 0.75°C/min | Final cooling step to transfer temperature, held at -60°C for 15 min before transfer |
After a minimum of 24 h storage, samples were thawed rapidly in a VIA Thaw CB1000 unit (Cytiva) and recultured. Viable cell count, through fluorescein diacetate (FDA) staining, and metabolic activity, through resazurin sodium salt reduction, was assessed at 24, 48, and 72 h post-thaw using a Cytell Cell Imaging System (Cytiva) and GENios FL plate reader (Tecan), respectively.
Thermal tests were carried out with cryobags containing 70 mL cell-free cryopreservation medium. In one test, a t-type thermocouple was attached externally to each of the eight cryobags, and data recorded during cooling, to assess inter-sample variability.
In a second test, the effects of placing a bag horizontally or vertically in a VIA Freeze system were assessed. One cryobag was placed in a horizontal plate (Cytiva) on one half of a VIA Freeze Quad unit with a thermocouple attached (Fig 2A). On the other half, four 70 mL cryobags were placed in cassettes held between vertical plates in a rack set-up (Fig 2B) with four thermocouples attached to separate points of one bag (Fig 2C) to also assess intra-sample thermal variation.
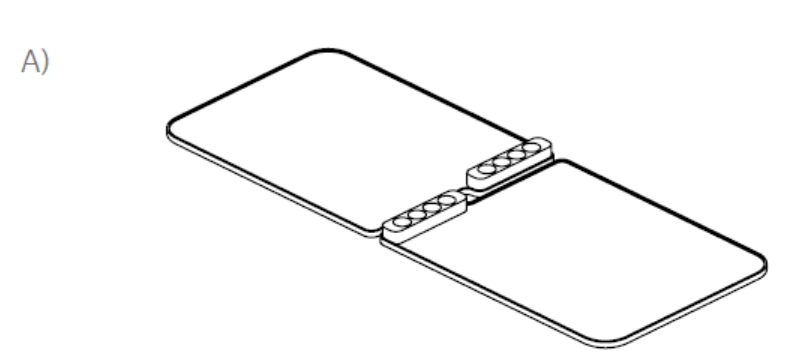
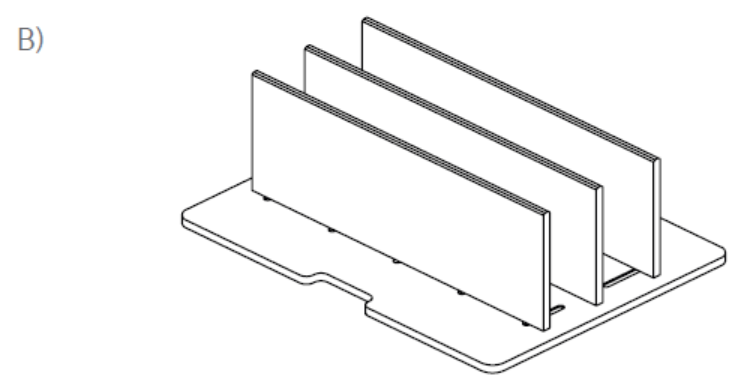
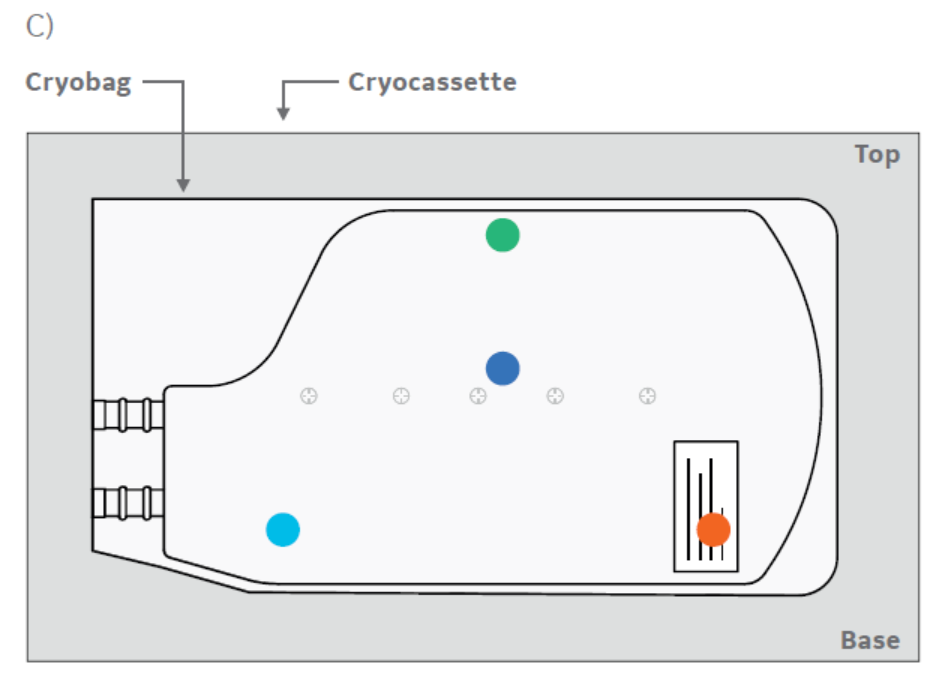
Fig 2. VIA Freeze cryobag plates for (A) horizontal and (B) vertical orientations. Vertical plate is adjustable to fit all cassette widths. (C) location and set-up of the cryobags during vertical freezing. Note that the larger volume of the bag is placed towards the base (i.e., sample plate) of the VIA Freeze sample area. Four thermocouples are attached to separate points on the cryobag (colored dots).
Results
Consistency between samples cryopreserved in large volumes
An evaluation of post-thaw viability and metabolic activity was necessary to demonstrate the validity of the set-up for freezing cells in large volumes and the consistency that can be achieved across multiple samples.
Thermal profiles indicated that all cryobags in this large volume set‑up experience similar cooling profiles throughout cryopreservation (Fig 3).
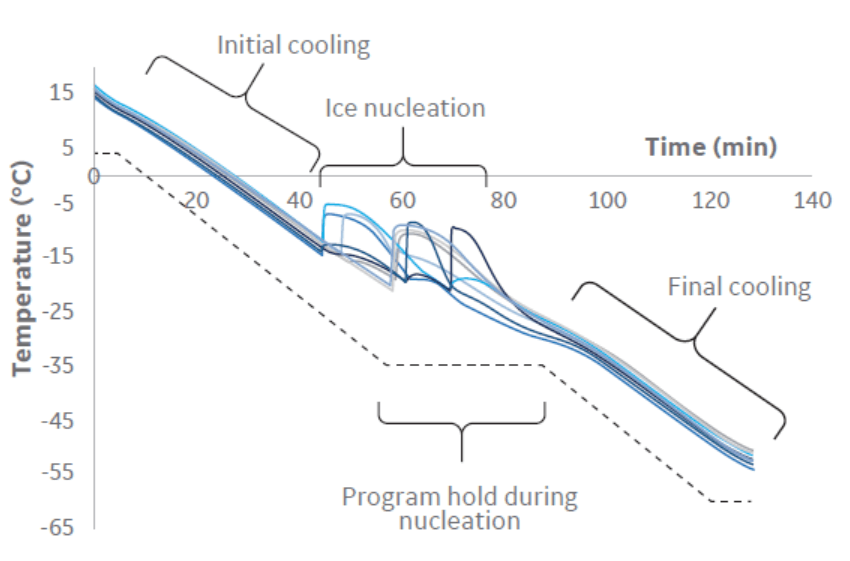
Fig 3. Cooling profile (dashed-black) programmed during the large volume cryopreservation, as well as the bag temperatures (blue) recorded in each of the eight cryobags filled with 70 mL volume each.
In turn, cells from all bags showed consistent recovery, with no significant effects on post-thaw viability or metabolic activity (Fig 4).
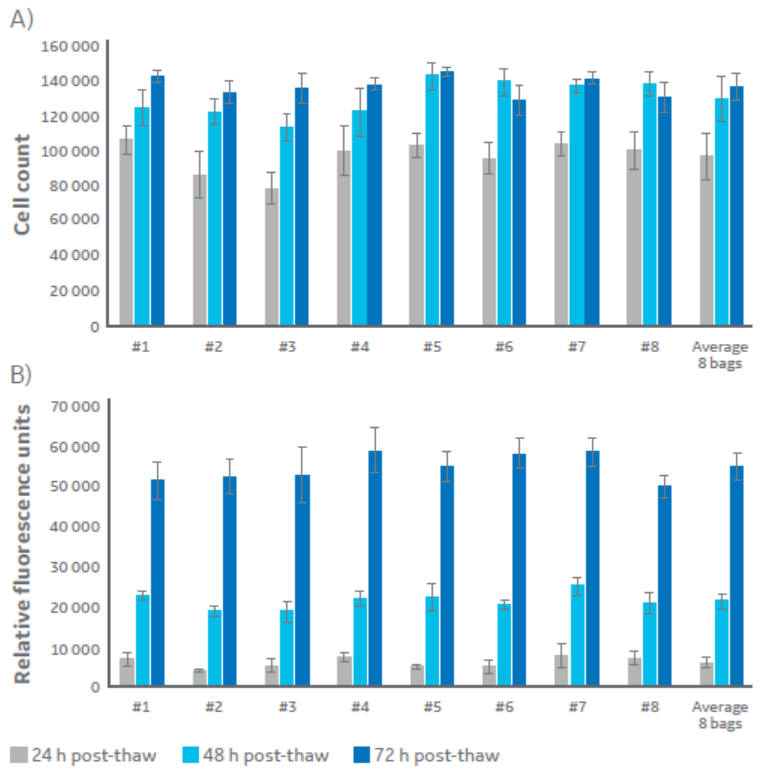
Fig 4. Analysis of samples from each of eight bags and average of bags. Two-tailed homoscedastic student’s t-test shows no significant differences between the pairs (P<0.01). (A) viable cell count via fluorescein diacetate staining, and (B) metabolic activity read through resazurin sodium salt reduction. Cells were thawed then cultured under normal conditions for 24, 48, and 72 h. Data is n=5 technical replicates ± one standard deviation. Average of the eight bags (far right) is n=8 ± SD).
Consistency between vertical and horizontal cryopreservation
Conventionally, approaches for the cryopreservation of large sample volumes involve laying cryobags horizontally within a controlled-rate freezer. This limits the total volume that can be loaded in one cooling cycle. This study compared the physical and biological impact of cryopreserving bags horizontally and vertically. The vertical set-up enables cryopreservation of multiple cryobags simultaneously, making more efficient use of the cooling space available.
Figure 5 indicates that there is no significant difference in post-thaw cell viability between samples cryopreserved in vertical and horizontal plates. There is also little difference in thermal profile between samples frozen horizontally and vertically, with multiple bags placed vertically having the same thermal profile as only one horizontal bag (Fig 6). In addition, Figure 6 demonstrates that there is minimal intra-sample thermal variation.
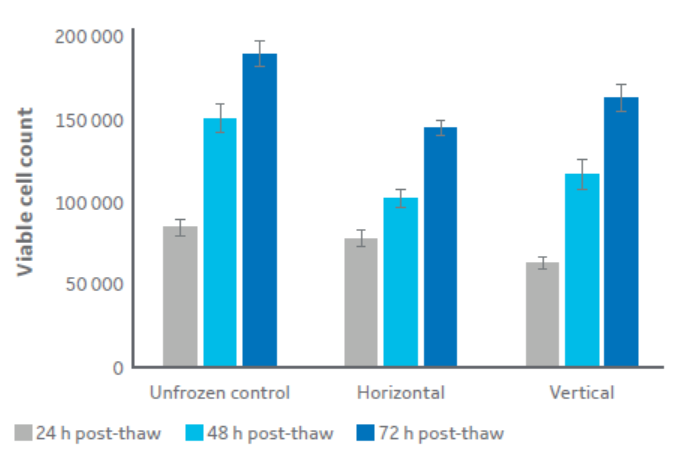
Fig 5. The viable cell count recorded for bags frozen in a low-volume horizontal set-up compared with a large volume vertical set-up, as well as unfrozen control. Cells were thawed then cultured normally for 24, 48, and 72 h. Two-tailed homoscedastic student’s t-test shows no significant differences between the cryopreserved conditions (P<0.01). Data is n=5 technical replicates ± one standard deviation.
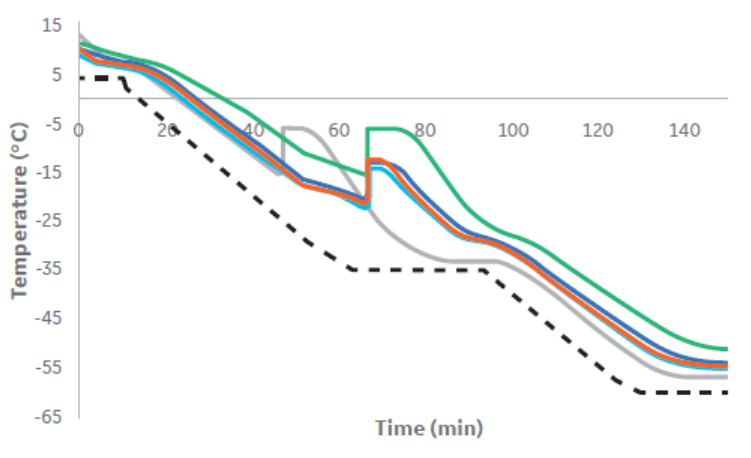
Fig 6. The thermal profiles observed when cryopreserving either horizontally (gray), or vertically (light blue, dark blue, orange and green, relating to thermocouple positions in Fig 2C), as well as the differences in thermal profile seen within a vertically cryopreserved bag. The programmed cooling profile is shown as black-dash.
The VIA Freeze Quad system achieves these consistent thermal profiles by detecting the sample load automatically and applying an equal cooling profile, regardless of volume loaded. This approach makes sure that each bag is cooled consistently from the base, and is not insulated by other bags in the system as is often the case in liquid nitrogen systems. Enabling consistent cooling profiles between bags and between different cooling cycles, as demonstrated in this study, is essential for reliable recovery of cellular products.
Conclusions on multi-bag cryopreservation without liquid nitrogen
This study demonstrates that the VIA Freeze Quad controlled-rate freezer enables cryopreservation of large volumes (up to 560 mL) with high and consistent post-thaw recovery between all samples. The orientation of the cryobags during the freezing process (vertical or horizontal) made no significant difference to post-thaw cell viability.
The system provides consistent cooling profiles, rates, and biological outcome regardless of volume used, allowing cryopreservation of large volumes of precious biological samples in an LN2-free process. This approach provides convenience and flexibility for making the most efficient use of the cooling space, with the added benefit of removing LN2-related sterility and safety concerns.
The set-up and cooling program described can also be used with smaller volumes and other cryo-containers to suit user requirements.
Ordering information
| Product | Product code |
|---|---|
| HyClone Calf Serum, U.S. origin | SH30073.03 |
| VIA Freeze Quad Controlled-Rate Freezer | VFQ_30010 |
| VIA Freeze multi-cryobag plate | ASY_30144 |
| VIA Freeze 250ml bag SBS adapters for Quad | ASY_30070 |
| VIA Freeze Quad 80 mm lid spacer | ASY_30025 |
| VIA Thaw CB1000 Dry Automated Thawer | TBA_30001 |
Read about controlled-rate freezing of cord blood cells.
Explore Cytiva solutions for cryogenic cold chain management.