Current state: islands of automation
In the biopharmaceutical industry (biopharma), process data is used in many ways throughout process development (PD) and the different production phases. But the pieces of equipment are often connected only by process; that is, the bioprocess material passing between each of them. Often this connection is discontinuous, meaning that equipment is controlled on an instrument level, which results in islands of automation. This article outlines how connectivity and a comprehensive automation solution can ease technology transfer and scaling from PD to final manufacturing scale. Suitable automation solutions also help maximize both manufacturing efficiency and facility utilization.
The need for data and data-driven decisions
The biopharmaceutical industry operates under a variety of business models. Some companies focus on either research, development, or manufacturing. Other companies carry out all these functions. Whatever the business model, effective business management is key in complex operations such as biologics production. The ability to deliver safe, effective, life-saving therapies to patients relies on data and decisions that are driven by this data.
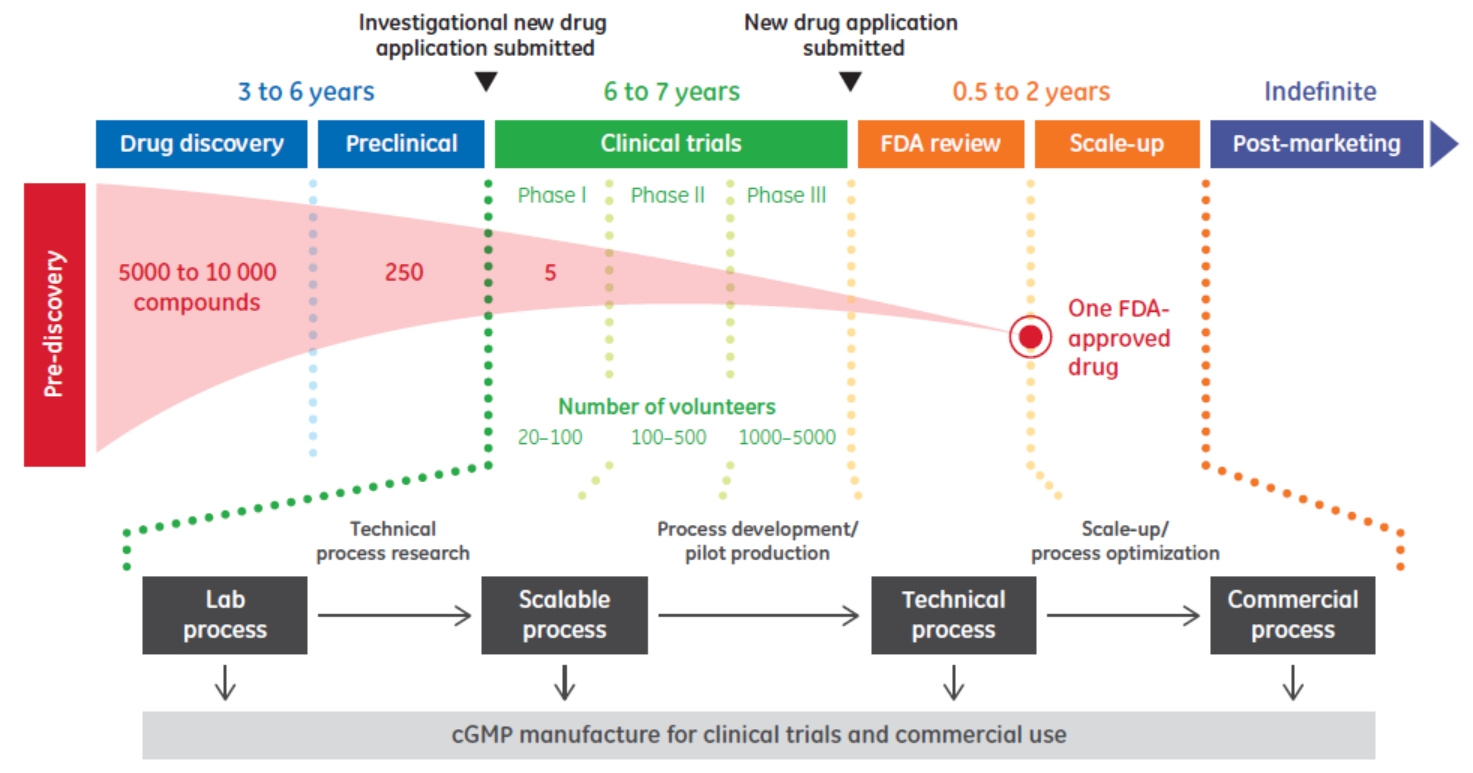
In biopharma, data is used in many ways throughout biologics development and production (Fig 1).
Fig 1. Overview of biopharmaceutical development and production in the United States.
Decisions based on the information derived from this data vary by time and phase. For example, there is scientific data associated with drug discovery and process data that can be used in scale-up. Manufacturing data is used to support regulatory compliance and plant efficiency. And there is market data on target population size. In highly regulated industries such as biopharma, data creates the foundation upon which quality and compliance are based. Automation is essential in order to acquire and manage data at each phase, from the supply chain to the boardroom. With comprehensive automation, data can be captured and used to support consistent biologics production that complies with regulatory guidelines.
Manufacturing typically focuses on one product with dedicated facilities. But process development activities often involve multiple parallel drug candidates and are spread across several sites. At the development stage, processes are characterized and improved by experimenting and adjusting at bench scale. As the process scales towards the volumes used in production, the effects of scaling on economic feasibility, product efficacy, and product quality are assessed. At this stage, where process conditions are set, it is important to keep processes versatile and experiments flexible. It is also important to make sure that the process continues to deliver product quality and yield comparable with previous scales. At the PD and scale-up stages, it is common to have proprietary measurement and control systems that are developed by the instrument supplier. Each instrument or piece of equipment also has its own instrument-level controller.
In PD, multiple parallel activities might be loosely or not at all integrated. But an efficient manufacturing operation requires tight coordination of functional groups, raw materials, procedures, and the unit operations that make up the bioprocess train. Automation plays a central role in manufacturing coordination and control. It is possible to maximize efficiency and facility utilization with proper automation architecture, especially by using an automation approach that integrates the different unit operations.
Process development and optimization
During development, process equipment and systems that meet the application requirements are selected from a range of available products. Then, process conditions need to be optimized. For example, cell culture conditions that maximize upstream productivity are established. And purification conditions that maximize the yield and purity downstream are determined. In addition, increasing demands from regulatory authorities for better process understanding is one of the cornerstones of the quality by design (QbD) initiative defined by the U.S. Food and Drug Administration (FDA). When supported by suitable process analytical technologies (PAT), data generated at this stage increases process knowledge. In turn, this knowledge results in a more efficient development process.
Design of experiments (DoE) is used to identify or screen input parameters that could affect the process output. Using a DoE approach, different process parameters can be varied at the same time. DoE allows the effect of each parameter, individually as well as combined, to be studied. Using DoE, sufficient information about a process can be obtained in a minimum number of experiments. A large amount of data is also created, which can serve as a basis for decision making.
Disjointed, or noncontiguous, development activities are a challenge for companies trying to move fast in the competitive biopharmaceutical market. Individual automated data collection and evaluation during PD help to reduce the time to market. But automation solutions that unify separate development activities are preferred to accelerate commercialization.
Bioprocessing equipment and automation
Ahead of regulatory approval and the manufacturing phase, a portfolio might contain many potential therapeutic drug products. To manage diverse portfolios, process development systems need to be versatile and able to handle as broad a process range as is practical. Compared with the manufacturing stage, the development stage is more tolerant of variability in process hardware and software (e.g., in process, data, operation, and documentation). In manufacturing, space and equipment are dedicated to a given product. But equipment used in PD might be preexisting from earlier development projects or new. If a piece of equipment used in development was originally designed with broad capabilities or can be easily modified, this can extend the capital investment.
Equipment used in PD is sourced from a variety of industry suppliers. The equipment has specific design features and attributes with little or no standardization between suppliers. Often, proprietary measurement and control subsystems are included. Proprietary subsystems, which are developed by each supplier, can be divided into:
- Custom design, which uses custom electronics (circuit boards, housings, etc.) and custom software (often in a format using firmware for deployment).
- Industry standard hardware components and off-the-shelf software development tools. This specifically refers to programmable logic controllers (PLC) or programmable automation controllers (PAC) and the software tools provided by PLC/PAC suppliers, such as GE Fanuc, Rockwell/Allen-Bradley, and Siemens.
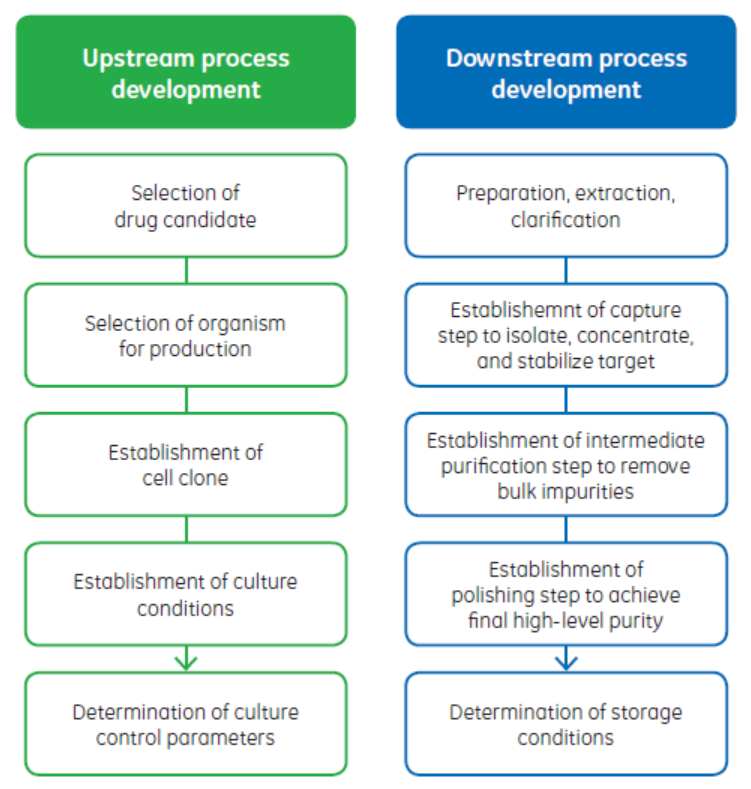
In automation terms, these proprietary measurement and control systems are at the instrument, equipment, or unit operation level. The control systems might even be different between unit operation or equipment sizes. Also, each unit operation, whether bioreactor, filtration, or chromatography system, has its own instrument-level controller. Various pieces of equipment are connected only by process, that is, the bioprocess material passing between each of them. This connection is often not continuous, and development activities are physically separated between upstream and downstream teams (Fig 2).
Fig 2. Process development activities are commonly dispersed between upstream and downstream teams. The parallel upstream and downstream PD activities might take place in different departments, buildings, facilities, or geographies
In addition to meeting objectives for process performance, data generated by the equipment must be available to be consolidated with data from other systems. The consolidated data unifies development activities that occur in different places, forming information from which to make appropriate decisions. Combining data for this purpose is left to the owner. Hence, systems must, at a minimum, be able to generate reports and allow export of data in a common format (e.g., tabular spreadsheet). Also, systems must have interface compatibility with other systems, using a standard for open platform communication (OPC).
Process scaling and transfer
The process at manufacturing scale needs to deliver equivalent purity and yield as the process during development. Biopharmaceutical process scale-up is generally complex, costly, and time-consuming. Upstream, scaling methodology is typically based on shear, tip speed, power, fluid velocity, among other factors. Points to consider downstream include column packing, hydrodynamic pressure drop, and efficiency of liquid flow distribution. A consistent geometry of production vessels, such as cell culture bioreactors or chromatography columns, streamlines work at this stage. But process parameters must be easy to transfer from one scale to the next. Modern system measurement and control software allows data to be exported in a spreadsheet-compatible format or exchanged with external systems using OPC. Use of these modern tools greatly eases transfer of the process from development to full manufacturing scale. Because methods, or recipes, and run data are installed in a network, they are easy to share between among functional groups or can be securely stored on a common database server.
Manufacturing automation for biologics production
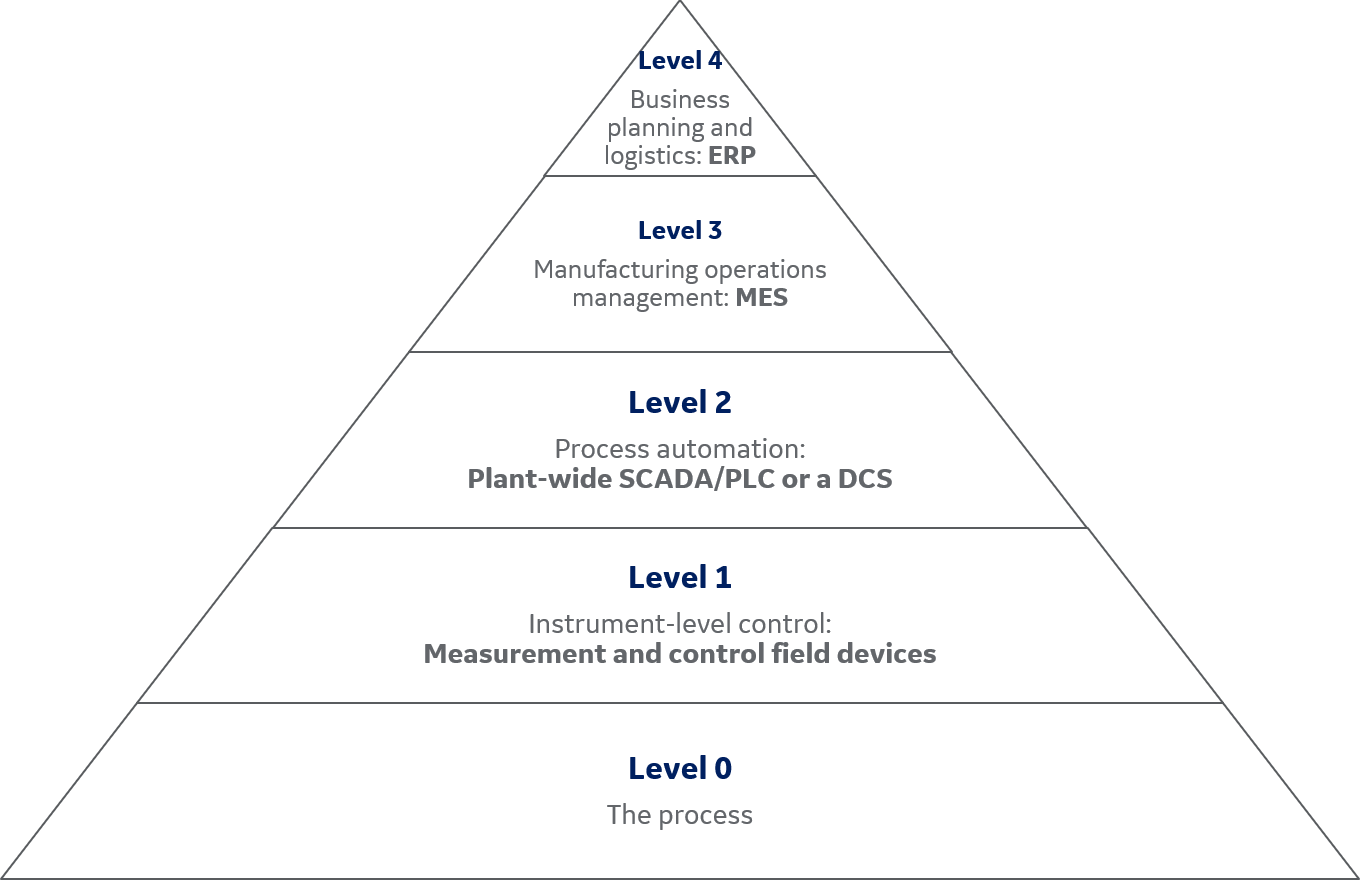
There are several unit operations in biopharmaceutical production. Upstream operations for traditional molecule types include preparation and storage of cell culture media and buffer solutions, plus multiple cell culture steps in different bioreactor technologies. Downstream operations for these molecules include protein purification steps using filtration and chromatography, plus concentration, buffer exchange, and conditioning steps. All these operations (through offline, manual, or semi-automated processes) create data sets that can facilitate batch release, process analysis, and application analyses. The noncontiguous activities often associated with PD are no longer acceptable. At the manufacturing stage, a highly integrated automation approach is required before comprehensive data management and operational efficiency can be achieved. With the appropriate automation architecture, it is possible to maximize process efficiency and facility utilization. When combined with other enabling technologies such as single-use components and systems, a properly designed automation architecture can facilitate multiproduct manufacturing. The S95 architectural model of the Instrument Society of America (ISA) may be used as a guide in reviewing the manufacturing process, the automation, and the data flow (Table 1).
Table 1. S95 control hierarchy
| Level | Control | System | Networking | Bioprocess-specific |
|---|---|---|---|---|
| 4 | Business planning and logistics: plant product scheduling, operational management, etc. | Enterprise resource planning(ERP) | Business process information network | |
| 3 | Manufacturing operations management: production dispatching, detailed production scheduling, production tracking, etc. | Manufacturing execution systems (MES) | Operations information network | |
| 2 |
|
|
| Batch control, including batch and fed-batch process types |
| 1 |
|
| Instrument buses, control networks, other specialized industrial device level networks | Unit operations:
|
| 0 |
| Unit operations:
|
ISA, an industry organization focused on instrumentation and control, has published several guidelines and standards for use in any industry where they apply. The ISA-95 standard includes three identified manufacturing types: discrete, batch, and continuous. Discrete manufacturing is associated with individual end products, such as a piece of equipment that can be individually identified by a serialized number. Batch, on the other hand, is associated with products made between specific process start and end times. And the product can be traced back to a specific production lot, or batch. In continuous manufacturing the individual products cannot readily be identified as separate units. One example of continuous manufacturing is oil and gas production.
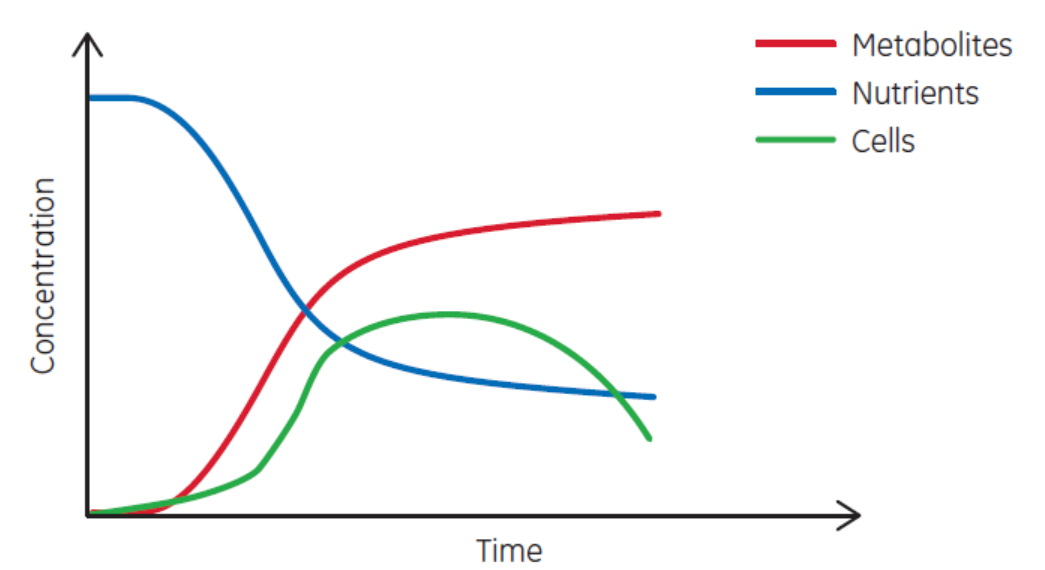
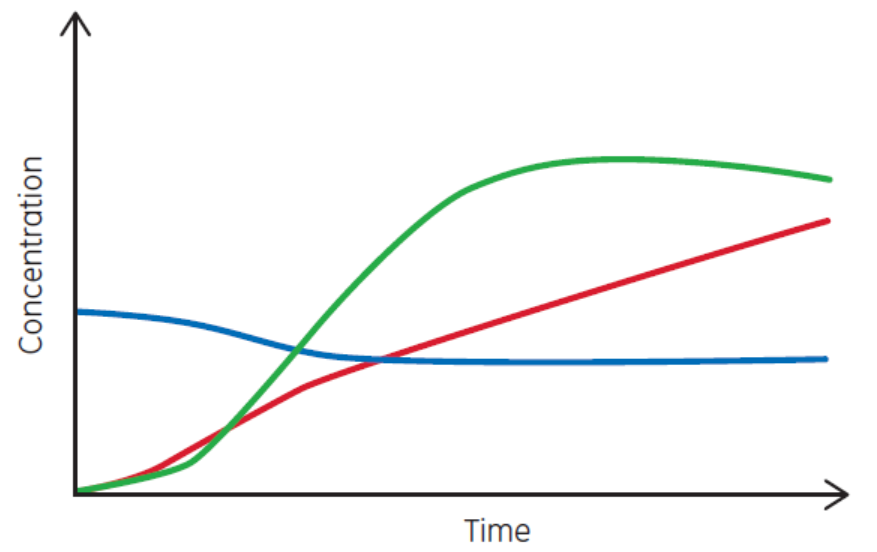
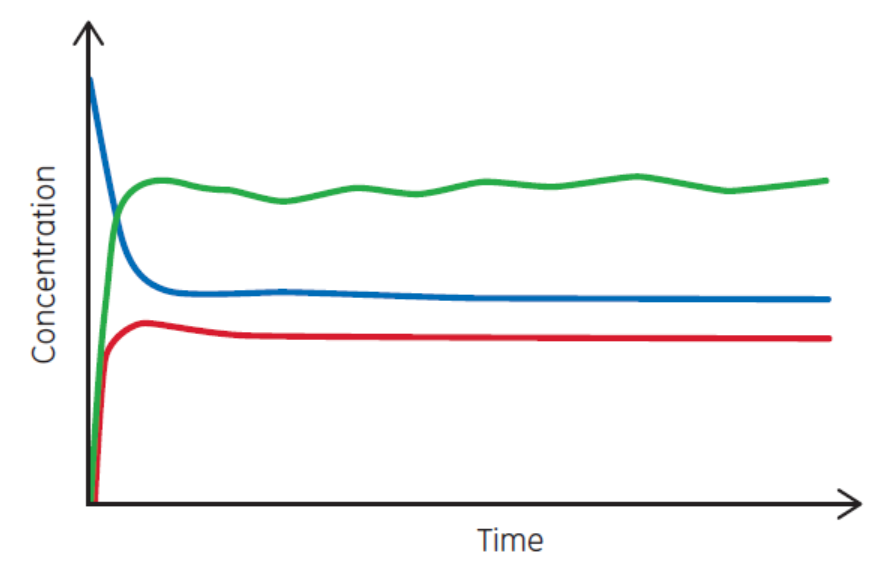
Most of biopharma is focused on what ISA defines as batch manufacturing. Under that category, the three common bioprocess types are batch, fed-batch, and perfusion. Each has a characteristic set of curves, as shown in Figure 3. Perfusion is the closest process type to continuous production. But perfusion has a definable duration, ranging from weeks to months.
(A)
(B)
(C)
Fig 3. Examples of (A) batch, (B) fed-batch, and (C) continuous processes. Perfusion is similar to (C) but has a definable duration.
ISA-95 levels with associated control and systems are summarized in Figure 4.
Fig 4. ISA-95 levels with corresponding controls and systems.
Level 0: the process
ISA-95 level 0 refers to the bioproduction process, for example, manufacturing of a vaccine, monoclonal antibody, or recombinant protein. Biomanufacturing is based on organisms, either cell culture or microbial. In batch manufacturing there is a finite growth period during which product is made. Regardless of how long the effective growth period is, at some point, the process is terminated, ending the batch. The automation architecture takes this production process as the basis for implementation at the next two levels (level 1 and 2).
Level 1: instrument-level control
ISA-95 level 1 relates to the individual unit operations and field devices to measure and control process parameters such as temperature, pH, DO, gas and liquid flow rates, weight, and pressure. Measurement field devices include probes and sensors plus their transmitters. The control field device category includes components such as pneumatic valves, peristaltic pumps, and agitator drive motors.
Level 2: process automation
Components that allow device communication support real-time transfer of process parameters between system control software and other plant-wide control systems, such as Rockwell, Emerson (DeltaV™), Honeywell, or Siemens systems. There are two basic ways to achieve process automation based on how the different unit operations are integrated:
- A plant-level SCADA system coupled to PLCs (SCADA/PLC).
- A DCS.
Implementation of SCADA/PLCs or DCS resides in ISA-95 level 2.
The SCADA/PLC scenario is a bottom-up, instrument-level approach. This scenario reflects the equipment supply chain’s variability as it relates to equipment automation practiced by the many suppliers. Each unit operation has the potential to represent a unique island of automation that has dedicated measurements (e.g., temperature, pH), field devices (valves, pumps, motors), and local controllers. Typically, a set-point value entered in SCADA is sent to the PLC, which compares the measured value to the set-point and controls the process to match the set-point value. SCADA/PLC-based automation platforms are often used to control batch or fed-batch processes.
The DCS is designed top-down. It relies on distributed networks of measurement and control devices. The DCS approach offers a more comprehensive, highly integrated automation approach that accounts for an entire manufacturing process. In this environment, separate unit operations do not require instrument-level control, such as a PLC. As a result, islands of automation are eliminated. Control is handled by the DCS, and only specific field measurements and control devices are needed at the equipment level. Typically, the DCS employs supplier-specific controllers (e.g., Emerson and Siemens) and both standard and proprietary protocols for communication (e.g., Foundation Fieldbus, PROFIBUS™, and DeviceNet™). Measured process values are transmitted to the DCS across instrument and control networks. When the measured value reaches the set-point, the DCS instructs, for example, a valve to open or close until the set-point level is matched. DCS-based automation platforms are commonly used to control both batch and continuous processes. Plant-wide control is a native function of the DCS. DCS platforms execute process-wide recipes, while SCADA/PLC-based platforms commonly execute recipes at the unit operation level.
Level 3: manufacturing execution system (MES)
A manufacturing execution system is used to monitor and document processes from raw material to finished goods. The MES captures process data and outcomes in real time to be used in decision making, for example, in how to improve process output. An MES is used among cross-functional groups, such as in production planning, detailed production scheduling, inventory, and production tracking.
An MES acts on ISA-95 level 3 to exchange data between the plant floor at level 2 and business planning and logistics functions at level 4. For example, data from level 2 is collected in a production tracking activity in the MES to generate a production performance report that is shared with the level 4 system. The information is interpreted at level 4. The output can be used in the MES to aid in optimizing use of resources such as material, equipment, and personnel in production scheduling.
Level 4: enterprise resource planning (ERP)
The enterprise resource planning system at ISA-95 level 4 offers an integrated overview of all production-related business processes. In the ERP system, data from different departments are collected, stored, and evaluated. This information is used in activities such as plant product scheduling and operational management. ERP systems facilitate sharing of information between different business functions and connection with external parties, for instance, with raw material inventory levels and their associated suppliers, and logistics companies responsible for finished product distribution.
Process intensification and the role of automation
Modern manufacturing concepts such as just-in-time (JIT), LEAN, and Six-Sigma all apply in biomanufacturing. Although biomanufacturing today has many requirements, some external (regulatory compliance, audit-ability, product efficacy, quality) and others internal (quality, reproducibility, cost of goods), the goal is to maximize facility utilization. At the manufacturing level, facility utilization is essential. Getting the most product from the equipment and space is a competitive advantage. Whether achieved through more product per batch, more batches over time, or a combination of both, former, less productive ways of working will no longer survive.
Process intensification is commonly perceived as a promising path to reduce costs and improve throughput when developing and producing biopharmaceuticals. One way to achieve an intensified process is to transition from batch to a form of continuous processing. In a continuous process, product is moving through the process steps in an uninterrupted manner. A continuous process allows use of smaller equipment and minimizes the need for intermediate hold-up tanks. As a result, the facility footprint can be reduced. Because process steps are interconnected, the overall process time can also be reduced. Hence, productivity can be greatly increased in continuous processing compared with in batch processing.
Closed system operation is another way of decreasing the number of process steps to reduce the required floor space and shorten the overall process time. Ready-to-use and single-use products support closed system operation because they are ready for immediate use, omitting the need for separate product preparation and validation. Ready-to-use products reduce the time for process setup and initiation. Adding single-use equipment to the process reduces changeover time between production runs, as disposables do not require cleaning and cleaning validation between batches.
A continuous process is maintained at steady state to keep quantities, such as feed input, temperatures, pressures, and output stream, within defined limits. This simplifies surveillance and control. Continuous processes are easily integrated in process automation workflows, which supports the PAT initiative defined by the FDA. Continuous processing using automated workflows also minimizes contamination risk and opportunity for operator error by decreasing manual handling between process steps.
Unit operations on modular equipment platforms make it possible to decouple the process hardware and the supplier’s preferred automation platform. This decoupling simplifies integration into comprehensive automation solutions. An automation solution that spans the complete upstream-downstream bioprocess train offers advantages. Such a solution under either the SCADA-PLC or the DCS automation scenarios can greatly facilitate data management, process control, and operational efficiency in continuous processing.
How to improve overall operational efficiency
For any given biotherapeutic, development and manufacturing are usually handled by different parts of an organization. Sometimes these functions are even handled by different organizations. Organizations vary in size, can have functions that are co-located or separated by geography, and might outsource various process stages. Hence, operational needs can be very different, with little in common but the bioprocess between them.
The equipment and automation gaps that often exist between development and manufacturing can be effectively closed with the proper choice of bioprocess equipment. A modular equipment platform provides the highest degree of flexibility. Turnkey (measurement and control included), modular systems are preferred in environments where little or no automation infrastructure exists. This scenario is common in development environments. In manufacturing, where a preexisting automation infrastructure is more likely to be present, such modular systems can be integrated with or without their measurement and control subsystems. The availability of open communication standards further eases equipment integration within both SCADA-PLC and DCS environments.
Selecting an equipment supplier that can provide multiple unit operations, each on modular product platforms, offers advantages. This choice combines the benefits described above with single-source responsibility for integration and operation, documentation, and support. Suppliers with product offerings in single-use equipment, along with associated consumables, provide even more options for configuring effective bioprocess development and manufacturing. In the development stage, the upstream and downstream systems can be scaled in concert. This allows physical interconnections and data management to be optimized. Also, the transition to manufacturing scale will be faster and smoother. At the manufacturing stage, the various unit operations can be looked at as a more highly integrated bioprocess train.
Equipment choice ties the bioprocess to the automation architecture. Choosing the right automation solution, especially one that spans the complete upstream-downstream bioprocess train under either the SCADA-PLC or DCS automation scenarios, greatly facilitates data management and process control. With data connectivity and a comprehensive automation solution, all functions such as procurement, finance, production, material management, quality and more can be aligned to improve overall operational efficiency.
Learn more about automation solutions for bioprocessing and cell & gene therapy.