Article inspired by a Tapas and TECH Talks digital event. Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) team members shared peer-to-peer insights.
The therapeutic landscape has been evolving rapidly over the last few years, driven by discoveries in biologic modalities. The increase in levels of complexity, compared to the history of bioprocessing, raises some key challenges in terms of manufacturing these multicomponent or multifaceted therapy types.
As the industry moves from small molecules and natural products into biologics, engineered cells and viral vectors, and ultimately to the future where we're manufacturing restorative or regenerative therapies in their entirety, we have a tradeoff between the simplicity of the process and how cost effective it is.
Looking to the future, autologous and allogeneic therapies are likely to coexist. Both therapy types must be cost effective and efficient to produce, in order to reach the many people they will serve. A combination of new methods and technologies will be needed to achieve these goals. Among the choices are continuous manufacturing methods, new adherent cell technologies, and adapting cells to grow in suspension culture.
An ongoing collaboration between Cytiva and CCRM in the Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) aims to evaluate the options and bring them forward to further industrialize cell and gene therapy processes.
This article focuses on custom media development borne of this collaboration. Here we describe our process, highlighting a case study to remove serum from the expansion step of an autologous cell therapy. The challenge is to do so in a cost-effective manner without affecting cell performance.
Why focus on media development?
One of the primary concerns for media developers and startup therapy providers is including serum in their base media. So, that's something we have worked to remove. Another reason to focus on media development is to tailor your media to your process and get away from proprietary formulations. And, of course, everyone's interested in reducing the cost of goods (COGs) of the process as well as defining and reducing the risk profile.
Approaches to media development
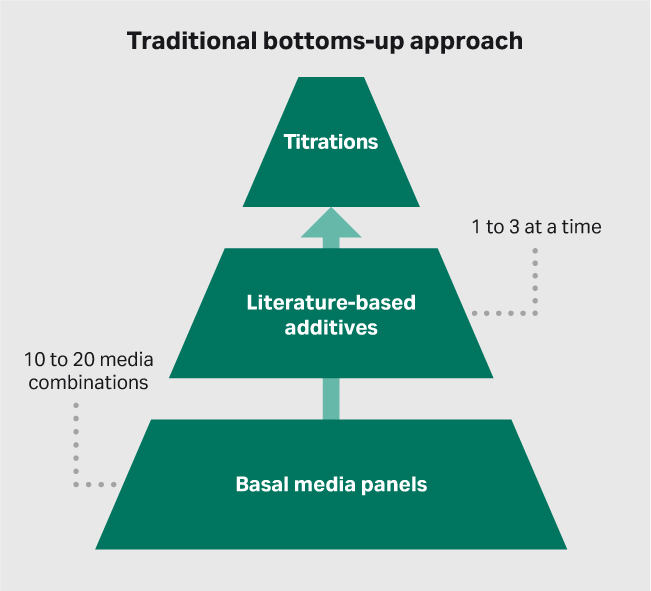
Media development has traditionally been a bottoms-up activity (Fig 1A). This approach looks at combining basal media panels with different additives, including lipid mixtures, cytokine mixtures, growth factors, etc. Using the literature as a guide, these additives are titrated for optimal performance in any given cell system. The bottoms-up approach is highly manual and suffers from low throughput, typically starting with no more than 10 or 20 base media panels and moving towards evaluating just 1 to 3 additives at a time.
(A)
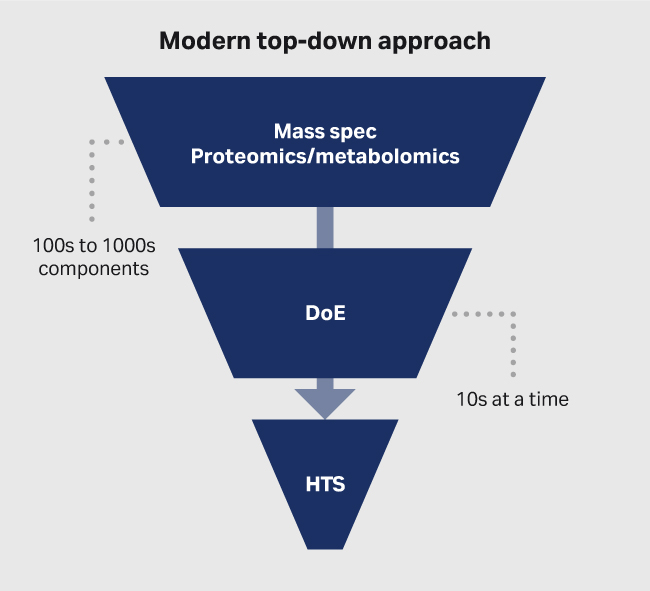
(B)
Fig 1. Approaches to media development. (A) traditional bottoms-up and (B) modern top-down approach.
At CCRM and CATCT we use the top-down approach (Fig 1B), which is based heavily on mass spectrometry (mass spec) proteomics and metabolomics. It's informed by design of experiments (DoE) screening, which then moves into high-throughput screens (HTS). The few formulations selected in HTS can then be validated at small and large scale. In contrast to the bottoms-up approach, thousands of formulations can be screened in parallel using advanced liquid handling robots.
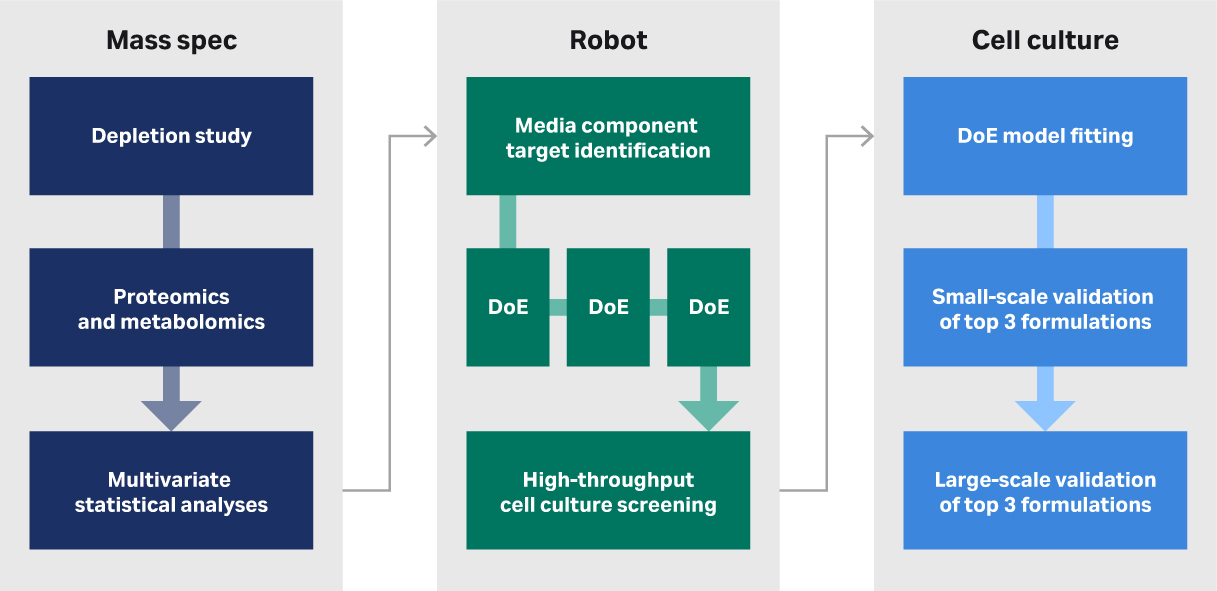
Figure 2 provides an overview of the top-down approach we use in our media development service.
Fig 2. Overview of steps in the top-down media development approach used at CCRM and CATCT.
Define the solution space
First, we aim to identify the solution space, which includes all the possible (i.e., candidate) media components that we could have in a custom formulation. Initially we use a fully supportive medium that probably contains serum. Through depletion studies and mass spec we determine which components of that medium are available to the cells as a function of time when the cells are grown under the conditions used to make the product. This helps us to pare down the solution space and the candidate components to look at.
For an autologous therapy, we want to look at the components with an impact across multiple apheresis donors. Conversely, if we are doing media development for a specific cell line, we may want to screen against various clones in that cell line process.
We group the components that have an impact across different conditions to reduce the level of background noise. That requires a deep dive into multivariate statistical analyses. This process allows us to take the complete solution space and narrow it down, again, to the effective components in the mix. Of course, this is informed by some intelligent data mining in the literature.
At the final phase of defining the solution space, we identify specific candidate components that have an impact on whatever we're screening for in the cell line. It might be growth, the ability to secrete a certain factor, or virus production. But the approach remains the same here. We get down to the specific components, noting which ones have a positive, negative, or neutral effect. From here we can start to drive toward effective concentrations that contribute to the quality that we're screening for.
Design DoEs and perform high-throughput screenings
The next step is to take our informed shopping list of components and set up some HTS to measure the impact of these components on the growth rate of the cells (or quality of interest). This part of the workflow relies heavily on our liquid handling robot, which can make the formulations. First, the robot makes stock solution plates and the formulations plates. The robot handles the many liquid transfers required to make these custom media. This is not something you would be able to do on the bench.
We develop a DoE, set up the screen, and end up with a matrix that we program into the robot. Our liquid handler is equipped with an integrated incubator as well as onboard analytics, bright field cell counting, and fluorescence and other plate-based assays.
The robot will aspirate the spent media. We then collect that and bring it over to the mass spec for analysis. The robot will then add fresh media on top of the cells, send the plate over to the plate reader for a confluency read or a fluorescence read, and then stick it back into the incubator. And so five to seven days later, we've got thousands of growth curves versus thousands of formulations. We can reduce the solution space more, remove some of the formulations, and adjust the concentrations to better define the ultimate formulation of the media.
Perform model fitting and validation studies
We look at the output from a screening experiment showing the individual components as a function of time. We remove the components that don’t change over time, to improve the model fit. We can then move forward and make additional predictions that allow us to test the candidates and narrow the field as we go through iterations of our DoE. We can look at varying the concentrations of the components and check the impact. More is not always better.
“Data must be readable, easily retrievable, and permanent. Automated testing and manufacturing systems controls have to be properly in place to assure data integrity.”
The last step is to take those favorite recipes that we've drilled down to and test them at a small and large scale. In our case study, we tested three candidates in triplicate versus a serum control in small plate-based growth comparisons. And then we performed the large-scale validation, which is closer to a commercial expansion protocol.
We ordered 10 L each of these 3 formulations through Cytiva’s rapid response production workflow. That allowed us to test them in the Xuri Cell Expansion System W25. And we were also able to get a sense of what the commercial manufacturing challenges might be, because we're making this in our GMP manufacturing facility, not in our controlled process development lab. Once we pick our final formulation, we can order it in larger quantities from Cytiva.
View the event video for details on this robust approach to media development and see how the case study turned out.
About Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT)
This work was performed at the Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) in Toronto, Canada. This center was conceived in January, 2016 as a joint collaboration between Cytiva, CCRM, and the government of Canada. It was tasked with developing the next generation of solutions for effective manufacturing of cell and gene therapy products to make these treatments more accessible to patients. The center includes associates with expertise in process development and optimization, manufacturing, and many other specialties. Learn more about Fast Trak services for cell and gene therapy.