Freeze drying, lyophilization, and cryodesiccation are all terms used to describe the process of removing water from a sample at low temperature. This low-temperature dehydration process maximizes product or sample stability and shelf life, maintains chemical or biological function, and enables easier transportation and storage compared to a cold chain.
The process has applications in assay kit development, where it enables room-temperature shipping and storage of reagents and complete assays. Sample stabilization by lyophilization enables the production of pre-dispensed, single-dose reagents, which help:
- simplify assay setup,
- increase assay robustness and reliability,
- reduce the risk of sample contamination
What is lyophilization?
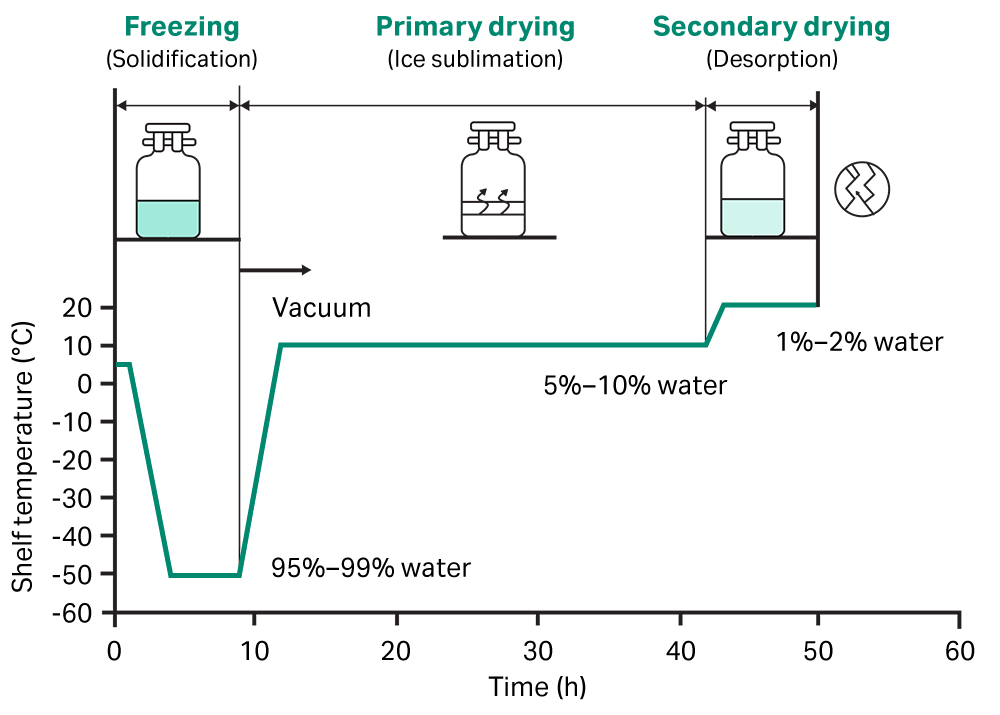
Lyophilization is a freeze-drying process (also known as cryodesiccation) that removes water from a sample or product by freezing and placing it under a vacuum (1). It relies on a phenomenon known as the triple point of water—the combination of temperature and pressure where the three phases of water (solid, liquid and gas) can co-exist and are equally stable. The lyophilization process typically involves three stages (Fig 1):
- Freezing
- Primary drying
- Secondary drying
A product or sample is first frozen with cryoprotective excipients, either in a dedicated freeze dryer or in a freezer at temperatures of approximately -40°C (below the triple point of water). Excipients provide a layer of protection during the freeze-drying process and help maintain stability of the intended function over time. The optimum combination and concentration of excipients are unique to each product or sample.
Gradual freezing would usually provide maximum efficiency for lyophilization, this forms large ice crystals. However, the large ice crystals can disrupt and damage materials that are structurally sensitive, such as cells. In these cases, the freezing step employs a rapid cooling profile to minimize ice crystal size.
The primary drying phase involves reducing the pressure of the surrounding environment below that of the triple point of water. The application of a small amount of heat then energizes the free ice crystals to undergo sublimation, transitioning directly from a solid to a gaseous phase.
This step removes approximately 95% of the water and results in the product or sample transforming from a glassy frozen state to a largely dry powder.
The final step removes any remaining unfrozen water molecules adsorbed to the product or sample. The temperature is increased slightly, disrupting interactions between water molecules and the frozen material, removing them through desorption.
The result is a lyophilized powder that is much lighter in terms of mass and smaller in volume than the original product or sample. Readying this lyophilized powder for use then simply involves adding sterile, distilled water or an appropriate buffer to form a solution in a process called reconstitution.
Fig 1. The process of lyophilization, involving three stages that remove water from a sample by freezing it and placing it under specific vacuum pressures and temperatures.
The challenges in long-term sample storage
Often, samples that require long-term storage in the biotechnology and pharmaceutical industries are highly labile. Application of heat for dehydration can, therefore, have consequences in terms of sample stability and quality.
Enzymes, for example, are used in many different assay kits and are often dehydrated to extend shelf life and ease transportation. These proteins have precise macromolecular structures required for their specific biological function. Heat stress would disrupt the noncovalent bonds and interactions that maintain these structures and in turn disrupt the in vivo function.
Not dehydrating samples intended for long-term storage at all is also not a viable option in many cases. Water can act as a powerful solvent and experiences a dramatic change in chemical structure when frozen. As a result, it can break covalent bonds found in biological macromolecules and, because the solid structure differs vastly from the liquid, those molecules can precipitate.
How lyophilization addresses sample storage challenges
Although it is a complicated process, lyophilization has become standard practice in many industries, including pharmaceutical, biotechnology, and agriculture. Given the adverse effects of heat and water, dehydration by lyophilization offers several advantages, from improved sample stability and purity to increased shelf life and reduced costs.
By removing the need to dehydrate by heating, lyophilization provides a convenient and safe method for long-term storage of lab samples and pharmaceutical products while preserving their activity.
As well as increasing shelf life, freeze-drying samples also reduces their weight and volume, helping cut down on shipping costs and environmental impact. Eliminating the need for shipping procedures designed to maintain sample stability, such as dry ice, also simplifies logistics and helps further reduce costs.
These benefits are especially advantageous when transporting samples and assays to developing countries with limited facilities and budgets. Long transportation distances and often hot and humid environments would otherwise put a substantial strain on cold chain transport and storage. With lyophilization, a temperature-controlled chain is not necessary.
An example of this can be found as long ago as World War II, which saw one of the first use cases of freeze-drying as a commercial technique(2). Lyophilization was used to render blood plasma and penicillin chemically stable and viable without refrigeration. As a result, both could be transported to their intended recipients regardless of cold chain transport availability. This process is still in use today.
A complete, stabilized assay in a single tube
In addition to the practical and logistical benefits to shipping, costs, and shelf life, lyophilization offers scientists reliable, efficient, and accurate research tools in the form of individual reagents and complete, ready-to-use formulations in assay kits.
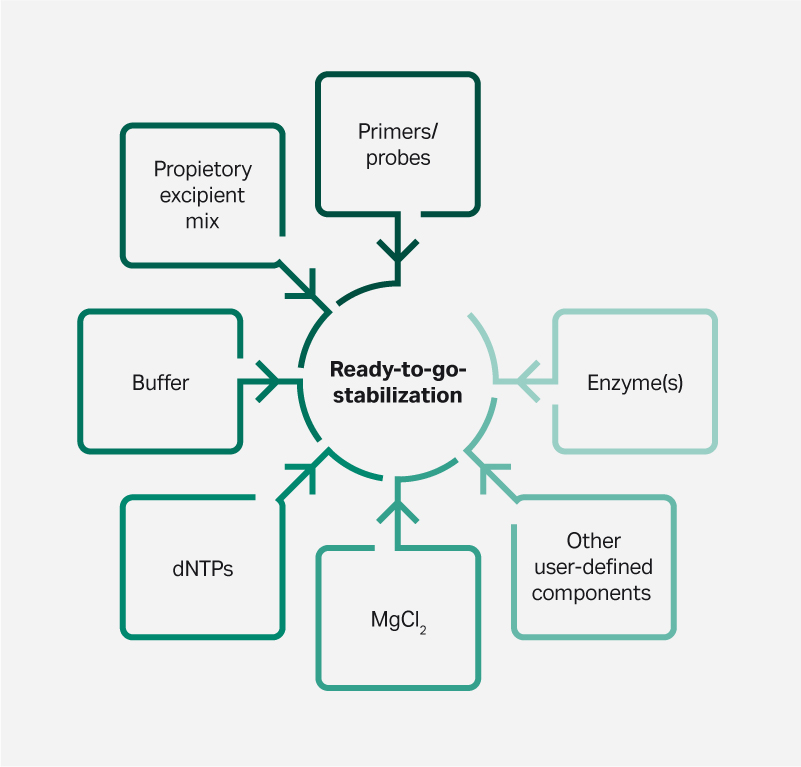
Through lyophilization, the individual components of an assay kit can be combined in a single tube. The result is a pre-dispensed, single-dose reagent that vastly simplifies assay setup (Fig 2).
In many cases, and particularly for pharmaceutical and biological products, excipients will be critical in formulations to help maintain the stability of the macromolecular structure (1, 3). Enzymes, for example, are still susceptible to destabilization during dehydration, losing activity even without the addition of heat. Excipients, such as amino acids and non-aqueous solvents, can act as cryoprotectants, minimizing this dehydration stress.
Using this lyophilization approach, researchers do not need to individually pipette microliter quantities of potentially expensive assay reagents, greatly simplifying their workflows. Fewer pipetting steps and less sample handling also help minimize training requirements, reduce costs and save time. The robustness and reliability of an assay are also improved along with data quality, and the risk of sample contamination is minimized.
Fig 2. A complete assay in a single tube. Example of components for an amplification-based custom Lyo-Stable formulation.
Custom Lyo-Stable formulation services and contract lyophilization
It is often not practical for laboratories to perform their own lyophilization as it requires a large capital investment with the purchase of complex equipment, including the freeze dryer itself. Obtaining the intended chemical or biological integrity and activity with every batch is also a challenge. Each product and sample is unique, requiring an optimized freeze-drying cycle and excipient formulation, which can take time and effort to determine.
The Lyo-Stable custom stabilization service from Cytiva enables researchers to avoid these expensive outlays and still benefit from all the advantages that come with lyophilization. The service uses proprietary excipients to deliver reagents and assays that are fully stable at ambient temperatures for several years. This has enabled companies to improve cost efficiencies and the shelf life of their products, and access challenging markets, such as labs and field researchers in developing countries where cold-chain transport and storage are difficult.
Utilizing contract lyophilization services removes the burden of excipient and process optimization from the researcher, freeing up valuable time and resources. The Lyo-Stable service provides the expertise and experience gained from Cytiva’s long-established Ready-To-Go lyophilization technology. The dedicated customised solutions team, with specialist knowledge, equipment and ISO-certified manufacturing capabilities, makes sure the objectives are met with every batch.
The benefits of Lyo-Stable lyophilization across industries
From consistent product quality to reduced costs and time savings, Lyo-Stable freeze-drying technology has many benefits. Table 1 provides a full summary.
Table 1. Summary of the benefits of lyophilization*
| Type | Benefit |
| Boost product quality | Provides batch-to-batch consistency with all samples treated uniformly. |
| Products are stable at a wide range of temperatures and so can be used in all climates and conditions while maintaining sample integrity. | |
| Provides up to two years room temperature stability with no detectable activity loss. | |
| Supports applications in the field, including remote or poorly accessible locations with insufficient infrastructure (e.g. developing countries) without affecting sample or data quality. | |
| Improves data quality and reliability of results while also reducing contamination risks through a pre-dispensed, single dose assay. | |
| Simplify workflows and save costs | Requires less sample handling and fewer pipetting steps to simplify end-user workflows. |
| Reduces training requirements as the assay or sample is ready to use. | |
| Compatible with downstream applications and automation. | |
| Increases shelf life resulting in less waste. | |
| Reduces transportation costs through the ability to ship without refrigeration. | |
| Reduce environmental impact | Reduced weight and volume make lyophilized samples ideal for shipping. |
| No dry or wet ice needed for transportation. | |
| Shipping is less hazardous than for wet reagents and doesn’t require special licenses. |
* Based on Cytiva's standard Ready-To-Go portfolio
From simple buffers to multi-component kits, Cytiva’s Lyo-Stable service can develop and manufacture custom products in small or large batch sizes depending on requirements. Custom manufacturing for genomics includes contract assay services and custom reagent services that can simplify customer workflows and supplement existing capabilities.
The Lyo-Stable service provides access to expertise, equipment, proprietary excipients, ISO-certified manufacturing, and decades of experience gained from developing catalogue and custom lyophilized products based on well-established Ready-To-Go technology.
Find out more about custom services by contacting Cytiva Scientific Support or reviewing how the company supports customers from product concept through to commercialization.