What is mixed mode chromatography?
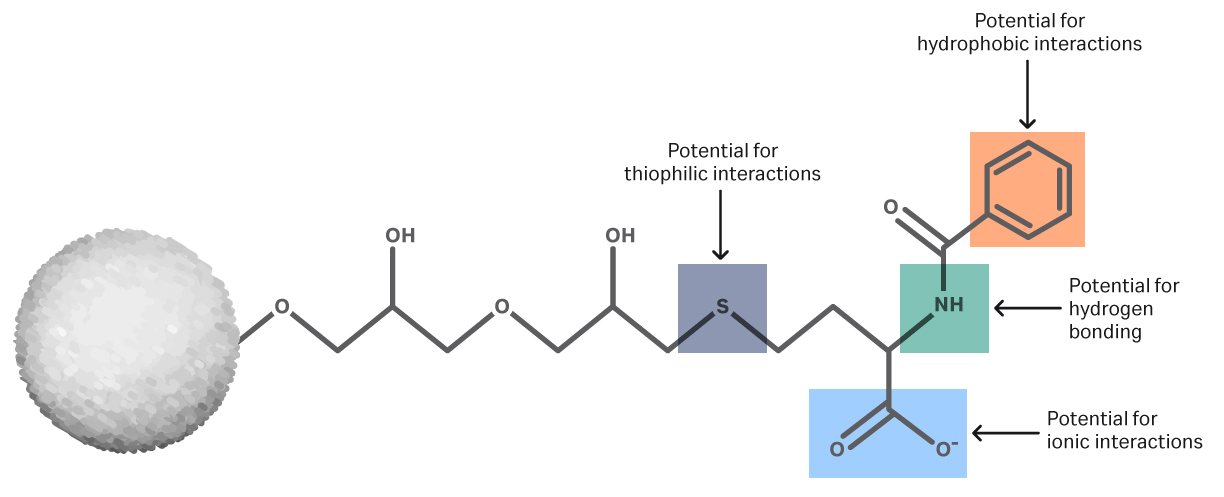
Mixed mode chromatography (or multimodal chromatography, MM) is a liquid chromatography technique used for the purification of proteins and other biomolecules. It can be successfully used in research, process development, and bioprocessing to purify biomolecules that are difficult to separate by other chromatography methods. In MM chromatography, ligands immobilized to the resin interact with the target protein molecule through multiple types of interactions where size exclusion, ionic, and hydrophobic interactions are the most important (Fig 1).
Mixed mode chromatography resins have a selectivity (the degree of separation of peaks measured at the top of the peak) that differs from that of “traditional” ligands seen in affinity chromatography (AC), ion exchange chromatography (IEX), and hydrophobic interaction chromatography (HIC). In other words, you can use MM chromatography to solve challenging purification problems that you cannot solve using AC, IEX, or HIC (Fig 1).
(A) Capto MMC resin
(B) Capto Core 700
Fig 1. Examples of multimodal chromatography resins: (A) Capto MMC — this resin is a mixed-mode cation exchanger with the properties of a weak cation exchanger. The ligand coupled to the resin surface allows electrostatic interactions as well as hydrophobic interaction, hydrogen bonding, or thiophilic interaction. The different possible interaction modes give the resin novel selectivity and and make it salt tolerant. This in turn allows sample to be loaded without dilution or a buffer exchange step, resulting in increased productivity.
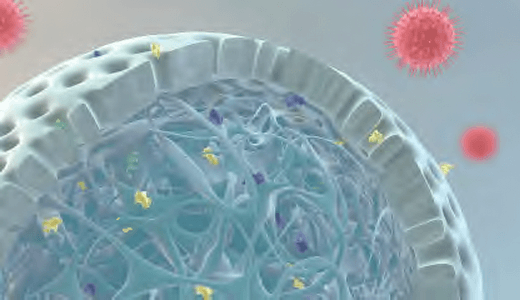
(B) Capto Core 700 — this novel resin combines size exclusion chromatography (SEC) and mixed-mode anion exchange characteristics. The schematic representation of the principle for Capto Core 700 shows a bead with the inactive porous shell and the ligand-activated core. Small proteins and contaminants (colored green, yellow, and purple) penetrate the core while target viruses (red) and larger proteins are excluded from the resin and
are collected in the chromatographic flowthrough.
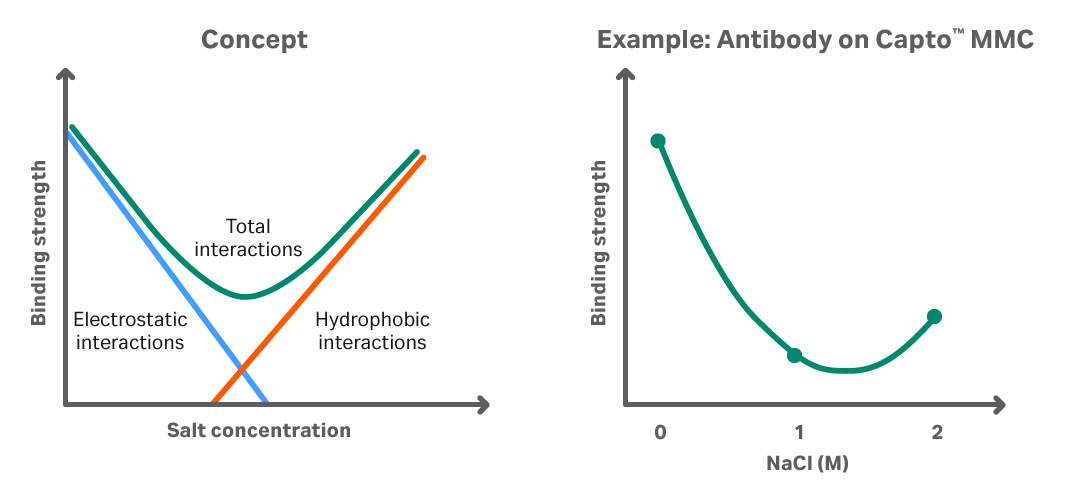
The strength of the individual interactions depends on the target molecule and on your overall process conditions (Fig 2).
Fig 2. Depending on the target protein properties and the conditions, different interactions can dominate. To the left, we see a schematic illustration of how electrostatic interactions decrease with increased salt concentration. At some point, hydrophobic interaction takes over and the binding strength increases again. To the right, we see an application showing how it translates to an antibody purification on Capto MMC resin.
Download our handbook for multimodal chromatography now
How does mixed mode chromatography work?
You can perform mixed mode chromatography either in bind/elute, or in flow-through mode. Table 1 shows how you use MM chromatography in four main steps in bind/elute mode. In bind/elute, the target molecule binds to the ligand coupled to the resin through mixed-mode interactions. Changes in the buffer composition and pH release the molecule from the resin to allow collection (the molecule “elutes” with the buffer).
Table 1. Four main steps of a mixed mode chromatography which you can perform in bind/elute mode
| Equilibration | Sample application and wash | Elution | Regeneration |
|
Prepare the column to the desired start conditions. When equilibration is reached, all charged groups of the stationary phase are associated with exchangeable counter ions as chloride or sodium ions. |
Target molecule binds to the ligand on the resin via multimodal interactions. The sample buffer needs to have the same pH and ionic strength as the starting buffer. |
The target molecule is released from the stationary phase by changing the buffer composition and the buffer pH. |
Removes all molecules still bound to the stationary phase.
|
In flow-through mode, impurities bind to the resin while the target molecule is collected in the chromatography flowthrough. See Determining optimal experimental conditions..
Watch our animation to easily visualize how mixed mode chromatography works:
Mixed mode chromatography can be advantageous for intermediate purification and polishing in a purification protocol/process. It offers you new options such as high conductivity binding and an alternative selectivity when conditions in the purification workflow are challenging.
Mixed mode chromatography offers you alternative solutions in purification workflows by widening the window of operation where traditional resins are not as effective as you had hoped. These circumstances may be encountered, for example:
- When the selectivity of traditional resin is insufficient to provide the required purity of the target protein
- If salt tolerance is required, for example, when the loading conductivity of the sample is too high for a traditional ion exchange resin
- When there is a need to reduce the number of purification steps
To explore examples of applications, see Application notes, posters and articles at the bottom of the page.
We recommend you explore a wide range of chromatography conditions early to increase process understanding and increase the likelihood of developing a robust purification process. This is the case for both traditional chromatography and MM chromatography.
When you compare an MM resin with traditional IEX and HIC resins, several questions may come to mind:
- What will happen on a multimodal resin that contains both interactions?
- Will the protein of interest bind in a high-conductivity environment?
- Under which conditions will it be possible to elute the target protein?
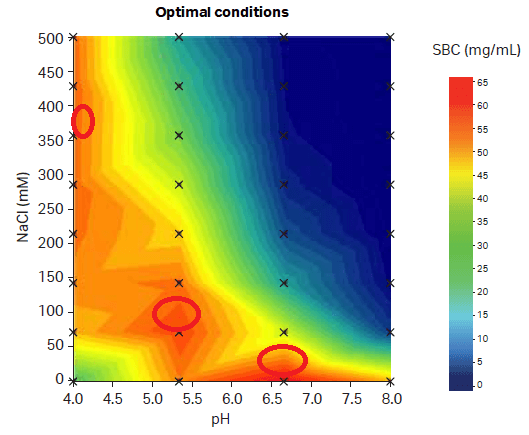
With an MM resin, it may be more difficult to predict the answers to these questions. The answers will be determined by the multimodal functionality of the resin, by the operating conditions, and by the target molecule itself. Optimization is therefore critical for success (Fig 3).
Fig 3. A contour map in a design of experiments (DoE) contour chart of static binding capacity (SBC). The contour map is measured under different salt and pH conditions on Capto MMC ImpRes resin. The map shows you that multiple optimal conditions are possible for this mixed mode resin. Unlike a traditional IEX resin, Capto MMC ImpRes can have high SBC even at high salt concentrations.
We provide additional recommendations for you in our MM chromatography handbook.
Bind/elute vs flow-through mode
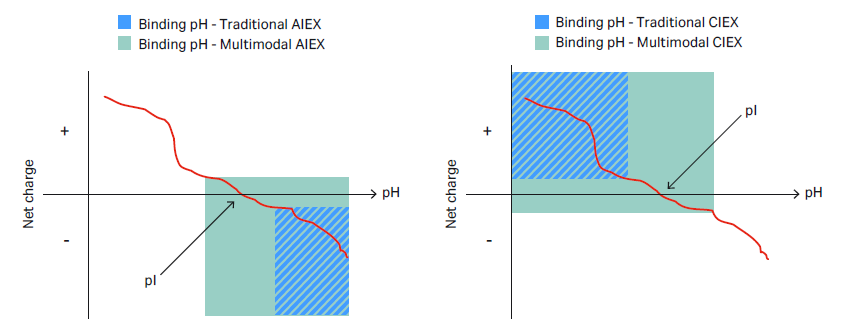
In MM chromatography, the choice between bind/elute and flow-through mode is more complex than when using a single method such as IEX alone. This is because multiple types of interactions occur in MM chromatography, and the strength of these individual interactions often depends on your overall process conditions. The pH range for binding is generally extended for a multimodal resin compared with a traditional IEX resin, which gives the MM resin novel selectivity and a wider operational window (Fig 4).
Fig 4. Net charge of a protein vs pH. Schematic illustration of the extended pH binding range for multimodal AIEX/CIEX (light green) compared with traditional anion exchange (AIEX)/cation exchange (CIEX) resin (light blue).
Because the isoelectric point (pI) of a protein is generally not a good indicator for choosing the correct pH for binding and elution with multimodal resin, screening of conditions is paramount. This can be performed with high-throughput formats, such as microplates or minicolumns. The experimental setup for screening studies should be performed using DoE, and the factors most typically screened for are pH and conductivity.
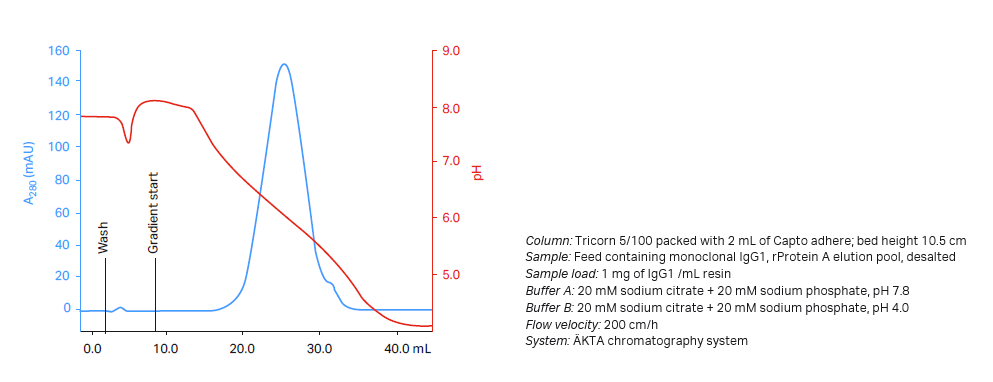
To help select the pH range to screen, you can perform a pH gradient elution experiment where an analytical amount of sample is loaded on a small column. The experiment will establish the elution pH of the sample. A salt gradient or a combined salt and pH gradient can also be run if you need better understanding of the mixed-mode behavior. An example is shown in Figure 5.
Fig 5. Establishing suitable conditions for DoE on Capto adhere in bind/elute mode.
Salt types and additives
Different salt types and additives can modulate the interactions of target molecule with MM resin.
Hydrophobic interaction is one of the interactions often involved, and your choice of salt may play an important role. Different salt types will affect the strength of interaction according to the Hofmeister series (Fig 6).
Typical ions used in HIC are found to the left in the series. The chaotropic ions to the right in the series, for example, iodine, reduce the hydrophobic interaction through the salting-out effect.
Anion: SO42- > HPO42- > acetate > Cl- > NO3- > Br- > ClO3- > I- > ClO4- > SCN-
Cation: NH4+ > K+ > Na+ > Li+ > Mg2+ > Ca2+ > guanidium
Fig 6. Hofmeister series. Organic solvents such as ethanol and isopropyl alcohol (isopropanol) decrease the strength of hydrophobic interactions. This can affect the binding of biomolecules to your MM resin.
Detergents and antifoaming agents such as Tween™ 80 can have a similar effect. Hydrogen bond disruptors such as urea and guanidium hydrochloride also affect the strength of interaction on your MM resin. Urea and guanidium salts are chaotropic and disrupt hydrogen bonds. Studies on several other modifiers, for example, amino acids or polyethylene glycol, have also been published.
- Screen pH and conductivity for optimal running conditions
- Use Design of Experiments (DoE)
- Consider parallel screening formats to speed up the process
- Choose appropriate chromatography resins:
- Capto MMC and Capto adhere for excellent flow properties
- Capto MMC ImpRes and Capto adhere ImpRes for optimal resolution
- Capto Core products for purification of viruses and other large target molecules
Cytiva offers multimodal resins in a wide range of formats to meet users’ needs at all stages of protein purification process development and manufacturing. All of our mixed mode chromatography resins (Table 2) are Capto resins, which are BioProcess resins developed and supported for production-scale chromatography.
Use our online resin selection tool for further selection guidance.
Table 2: Overview of resins for MM chromatography
| Resin | Main functionalities | Advantages | Use |
|---|---|---|---|
| Capto adhere |
|
|
Intermediate purification and polishing of mAbs after capture on protein A. Traditionally used in flowthrough mode. Purification of other target proteins from capture to polishing steps. |
| Capto adhere ImpRes | See Capto adhere above | Same as Capto adhere but with higher resolution and lower elution volumes. | Efficient mAb polishing, removal of aggregates and HCP, and separations of charge variants. Polishing resulting in smaller elution volumes. The properties of the small Capto ImpRes particle are best utilized in bind/elute mode. |
| Capto MMC |
|
Capto MMC gives high productivity and reduced cost with:
|
Capture and intermediate purification of proteins from large feed volumes by packed bed chromatography. Purification can be performed at the conductivity of the feed material. |
| Capto MMC ImpRes | See Capto MMC above | Same as Capto MMC but with higher resolution, lower elution volumes, and increased possibility to elute with salt only. | Efficient mAb polishing, removal of aggregates and HCP, and separation of charge variants. Polishing resulting in smaller elution volumes. The properties of the small Capto ImpRes particles are best utilized in bind/elute mode. |
| Capto Core 700 | SEC, AIEX, and HIC core-bead technology with ligand-activated core and nonfunctionalized shell. Allows efficient capture of contaminants while target molecules are collected in flowthrough. | Significantly improved productivity compared with SEC (100-fold). Straightforward optimization and robust performance. | Purification of viruses and other large target molecules. |
| Capto Core 400 | See Capto Core 700 above | Significantly improved productivity compared with SEC (100-fold) Straightforward optimization and robust performance | Purification of viruses and other large target molecules. Molecular Weight (MW) Cut-Off Mr ~ 400 000 |
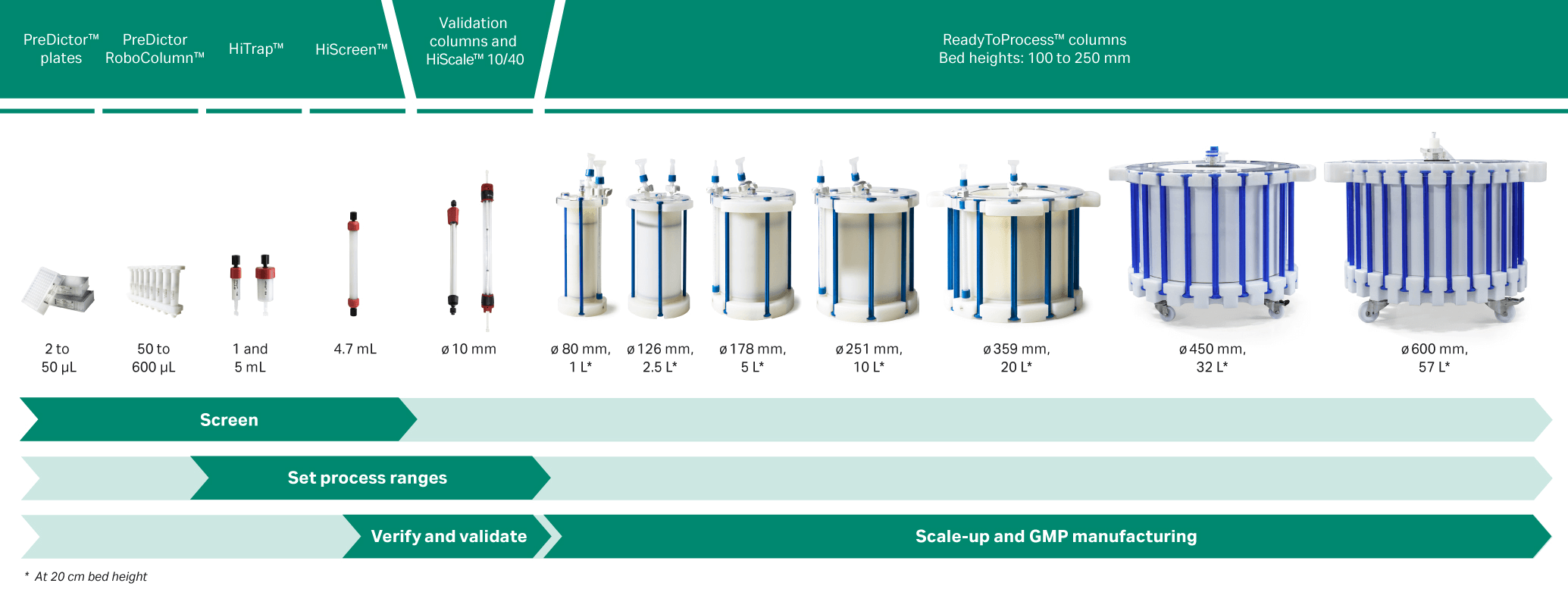
Our resins are available both in prepacked formats (see Fig 7) and as bulk resins to be packed in columns for use from lab scale to large-scale GMP manufacturing.
Fig 7. Overview of prepacked formats.
Monoclonal antibodies (mAbs), mAB charge variants, and biosimilars
- Purification of mAbs using modern chromatography resins and membranes
- Continuous chromatography in downstream processing of a monoclonal antibody
- Three-step monoclonal antibody purification processes using modern chromatography resins
- Tools and solutions for separation of charged mAb variants
- Exotoxin clearance from mAb samples in a two-step chromatography process
- Optimization of loading conditions on Capto adhere using design of experiments
- Polishing of monoclonal antibodies using Capto adhere ImpRes in bind and elute mode
- Polishing of monoclonal antibodies using Capto MMC ImpRes in bind and elute mode
- Selective removal of aggregates with Capto adhere
- Accurate comparability assessment of a biosimilar interferon in process development
Polysaccharides
- Efficient purification of meningococcal polysaccharides in a two-step chromatography workflow
- Efficient purification of pneumococcal polysaccharides in a chromatography workflow
- Efficient purification of Haemophilus influenzae type b capsular polysaccharide in a one-step chromatography workflow
Viruses and vaccines
- Accelerate flavivirus vaccine production with modern tools and solutions
- Downstream process development for efficient purification of adenovirus
- Manufacturing of viral vectors For use in gene therapy and development of therapeutic vaccines
- Ovalbumin removal in egg-based influenza vaccine production using Capto Core 700
- Purification of influenza A/H1N1 using Capto Core 700
- Scalable process for adenovirus production
- The use of Capto Core 700 and Capto Q ImpRes in the purification of human papilloma virus like particles
- Virus and exosome purification: harness core bead technology
Dab and Fab fragments
High-Throughput screening and DoE
- High-throughput screening and process development for capture of recombinant pro-insulin from E. coli
- High-throughput screening for elution conditions on Capto MMC using PreDictor plates
- Optimization of loading conditions on Capto adhere using design of experiments