Purifying tagged proteins
What is tagged protein purification?
Tagged protein purification uses affinity chromatography (AC) to purify recombinant proteins that have been engineered to include a specific peptide or protein sequence (tag). Common choices for protein affinity tags are polyhistidine (histidine-tag), glutathione S-transferase (GST), maltose-binding protein (MBP), Strep-tag® II, and FLAG™ tags. His-tagged proteins are purified with a variant of affinity chromatography called IMAC (immobilized metal affinity chromatography).
Why should I add a tag to the protein?
Tags simplify purification and enable scientists to use standard protocols. It improves the yield, purity, and solubility of the protein of interest, and minimizes the number of purification steps needed. You can also use tags for detection.
When should I use tagged protein purification?
You can use affinity purification of tagged proteins as the only purification step in applications that do not require very high purity. When you need very high purity – or purity between 95% and 99% – you can use this technique as the first (capture) step and follow it with a size exclusion chromatography (SEC) step. A tag might also increase the solubility of the target protein, prevent proteolysis, and increase protein detection.
When selecting the tag, consider that the tag can interfere with the structure or function of the target protein. That means you might need to remove the tag using a tag cleavage protocol. Adding a cleavage recognition sequence between protein and tag enables protease, a cleavage enzyme, to cleave off the tag after purification.
How does it work?
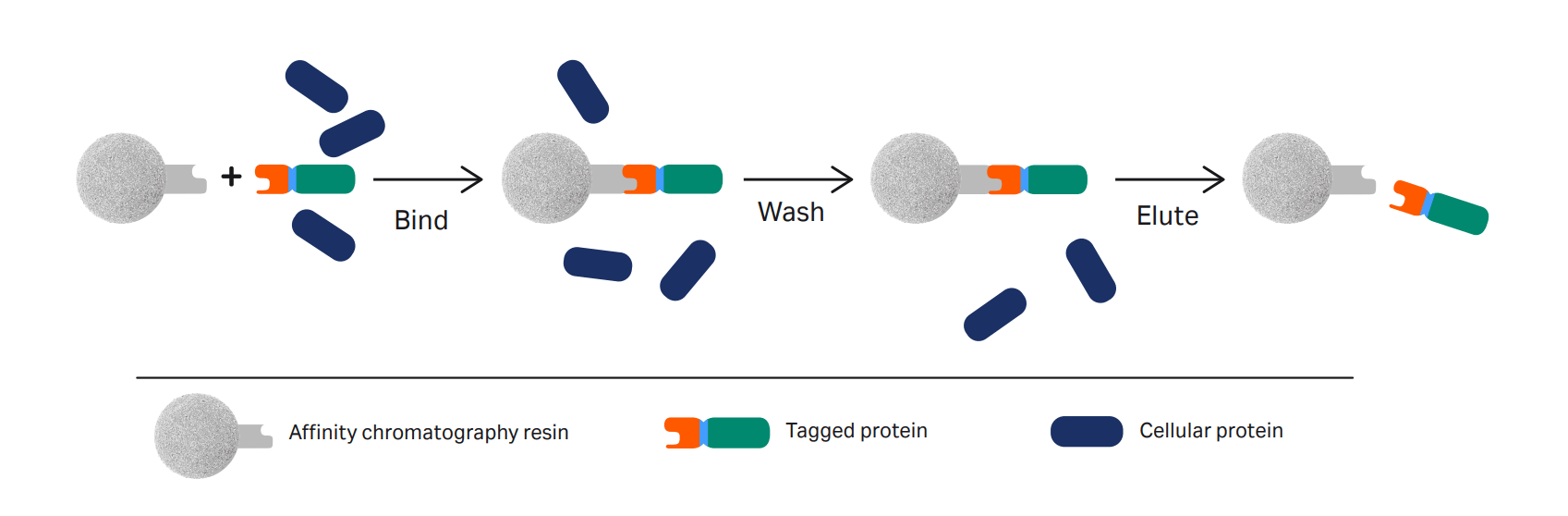
With an affinity tag attached, the target protein is specifically and reversibly bound to a chromatographic resin containing a binding substance (ligand) with affinity to the tag.
Tagged protein purification step-by-step
- Apply the sample to the column in conditions that favor the tag and ligand binding. Wash any unbound material out of the column.
- Elute the bound tagged protein. This is typically done using a competitive ligand.
- The eluted protein is usually at a high concentration. If you need to remove the tag before using the protein, you can perform cleavage using a site-specific protease.
Which affinity tag should I choose?
Adding an affinity tag to your protein can simplify purification and detection, and improve solubility and stability. You can also add two tags to the target protein – this is called dual tagging, and it can prevent proteolysis of the target protein. To perform dual tagging, position the two different tags on each end of the protein, and set up the purification protocols to ensure that a full-length of protein is purified.
If the tag interferes with the function or structure of the target protein, you can purify the native protein or remove the tag after purification instead.
If you decide to add a tag, considering a few factors will help you choose the right one. Think about the purification priorities, size of the tag, and cost of the chromatography resins. The table below shows some key characteristics of the four most used purification tags. His-tagged protein purification generally has the highest capacity, but can yield lower purity compared to other tags. The strep-tag gives a very high purity, but the capacity is lower. GST is somewhere in the middle, offering decent capacity and purity.
| His | Strep tag II | GST | MBP | |
|---|---|---|---|---|
| Size of tag | 6 aa | 8 aa | Mr 26 000 | Mr 40 000 |
| Protein binding capacity | 40 mg/mL | 6 mg/mL | 30 mg/mL | 10 mg/mL |
| Purity | + | +++ | ++ | ++ |
| Increased solubility | No | No | Yes | Yes |
| Risk for interference with function | Low | Low | High | High |
Get guidance on selecting the best tag for your protein.
Which expression system should I use?
You should select an expression system before setting up your purification protocol. Your options include bacteria, yeast, plants, insect, or mammalian cells grown in culture and transgenic animals or plants. Each host system has its own advantages and disadvantages. To decide, consider the amount and required purity of the protein, as well as its biological integrity and potential toxicity. For example, bacterial expression systems are not suitable if you need post-translational modification to produce a fully functional recombinant product. After selecting the host cells for expression of the target protein, you should select a suitable vector and know where the protein is expressed in the cell – along with what other host proteins are expressed in addition to the tagged protein. Different parts of the cell produce different amounts of host cell protein (HCP), and you need to remove HCP to obtain a pure protein. The host cell and location of the target protein in the host will both affect the methods for sample preparation, isolation, and purification.
Tips for tagged protein purification
- Identify the objectives of the final purified protein of interest. The experiment setup will determine the yield, size of purification products, and number of purification steps.
- Identify the required level of purity and ways to achieve it.
- Select the chromatography resin, format, and instrument that meet your needs.
- Determine whether you need to remove the tag and how.
- Ensure the sample is pure enough after SEC. If you observe several bands in the SDS-PAGE gel, optimize the AC step or add an extra intermediate purification step such as ion exchange chromatography.
Coomassie is a trademark of Thermo Fisher Scientific. FLAG is a trademark of Sigma-Aldrich Co. Strep-tag is a registered trademark of IBA GmbH.