Protein A capture chromatography is ubiquitous in mAb manufacturing for good reason: it’s a robust and broadly applicable operation to achieve high product purity in a single step. However, protein A resin cost can be a concern in early clinical stages, where only a few batches are produced and the full lifetime of the resin cannot be utilized. But there are ways to fine tune the capture step to better meet operational goals. In this article we’ll take a closer look at how input parameters influence protein A resin cost and productivity, using different Cytiva protein A resins and manufacturing scenarios as examples.
Biomanufacturers report that improving final product quality remains a central focus for their ongoing initiatives. Other high-priority efforts include maximizing manufacturing utilization, improving speed to the next milestone, expanding manufacturing capacity, and reducing the cost of goods sold (COGS).
All these factors are related to process productivity and process efficiency. But the impact of each on a given mAb capture process step depends on a variety of other factors: productivity of the upstream step, facility constraints, process scale, clinical stage, and so on (Table 1). In other words, there is no “one size fits all” mAb capture resin now, and that will be even more true in the future.
Table 1. Priorities for mAb manufacturing and their effects on the capture step
| Goal | Effect on capture step | Capture resin considerations |
| Increase productivity in upstream processes (i.e., higher titers) | Throughput must increase to avoid bottlenecks. | If the production bioreactor output is in batch mode, consider a resin with higher productivity. For continuous output (perfusion), larger columns may be required. |
| Improve speed to next milestone | Reduction of process development time when using platform processes and process modeling. Greater emphasis on scalable performance and getting it right the first time . |
Consider a resin with consistent quality backed with regulatory and application support. Good flow properties are key for large (commercial manufacturing-sized) columns to allow scalability and versatility for platform processes. |
| Reduce cost of goods | Need for resins with high productivity and durability. Reduced resin cost in low-demand manufacturing scenarios to mitigate limited lifetime utilization. |
High productivity will allow for reduced column volumes and processing duration, while durability will ensure long resin lifetime to reduce resin costs. In cases where the full lifetime can’t be utilized, such as in clinical production, focus should be on column cost, with commercial process feasibility and economy in mind. |
| Expand manufacturing capacity, maximize manufacturing utilization | Optimization of productivity while considering and alleviating facility constraints. | Resin selection can mitigate constraints such as buffer consumption, column dimensions, or flow rates. Non-productive time such as hold and change-over time can also be reduced to improve overall productivity. |
A toolbox of capture resins to support the needs in mAb manufacturing
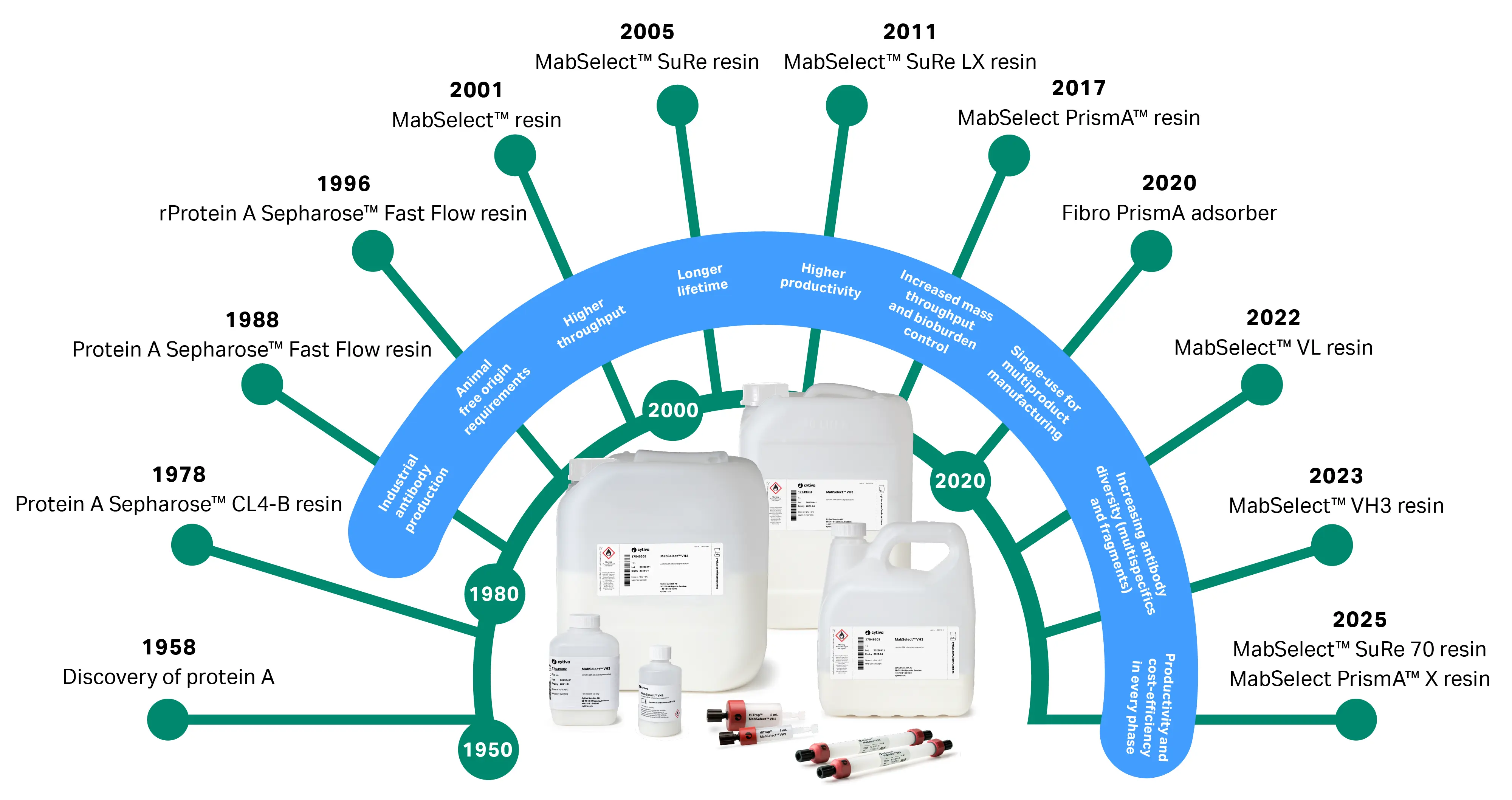
At Cytiva, we’ve been in the mAb capture space since 1978, continuously adapting and improving our resin offerings to meet market needs (Fig 1). Over the years it has become clear that the best capture resin for one process isn’t always the best capture resin for every process. Hence, what began as a single offering to leverage the potential of industrial antibody production has grown into a toolbox of resins for various production scenarios.
Fig 1. Timeline of market changes and corresponding protein A resin developments over the years.
In 2001, MabSelect™ protein A resin raised the bar for throughput and cleanability. More recent innovations put our protein A ligands on various base matrices and prepacked columns and addressed the unique challenges of bispecific antibodies and fragments. And most recently, we’ve introduced MabSelect™ SuRe 70 and MabSelect PrismA™ X resins, tailored to reflect the realities of clinical manufacturing and other low-demand processes.
A more tailored capture step
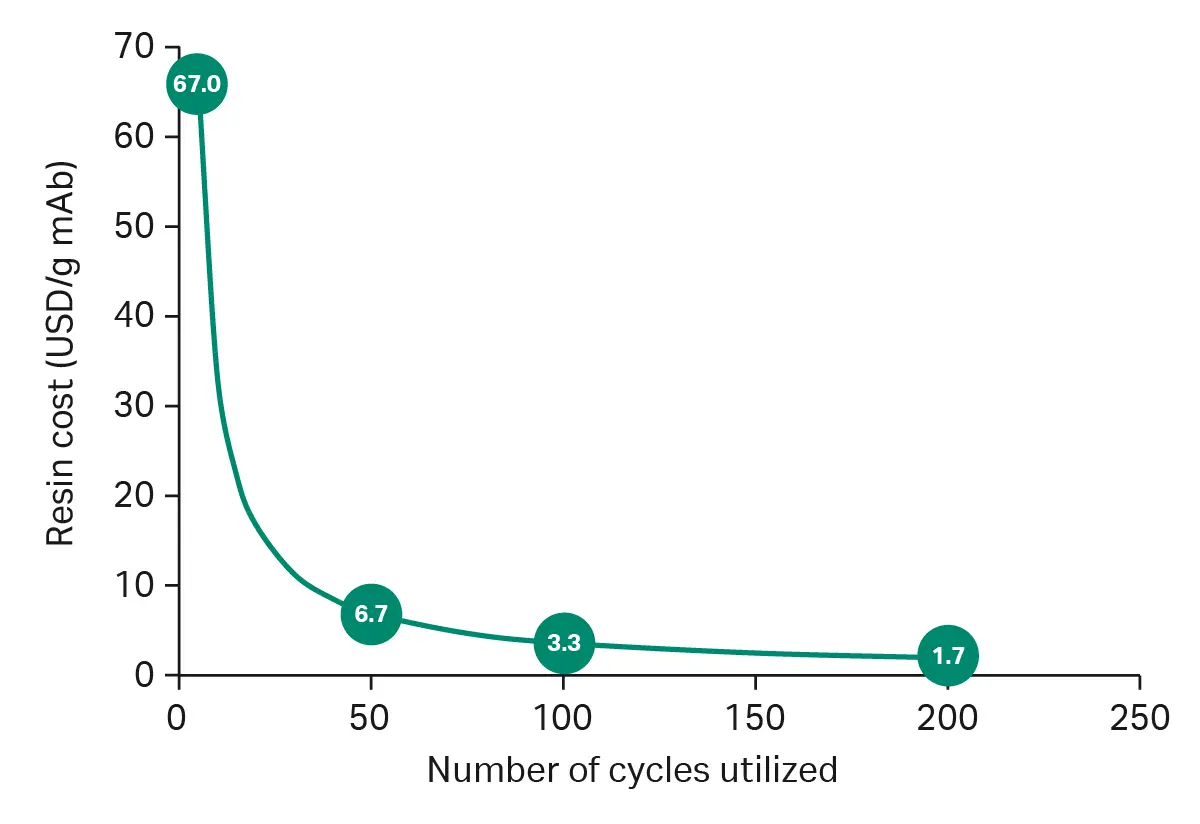
When only a few batches are produced, utilizing only a fraction of the full resin lifetime, the resin cost per amount of produced mAb will be high. Figure 2 shows an example of how resin cost per gram of purified antibody varies with the number of cycles utilized. For instance, at only five cycles utilization, the cost per gram of mAb may be around $67, whereas if we can use the resin for 100 cycles, the cost goes down to around $3 per gram.
Fig 2. Resin cost per number of cycles utilized.
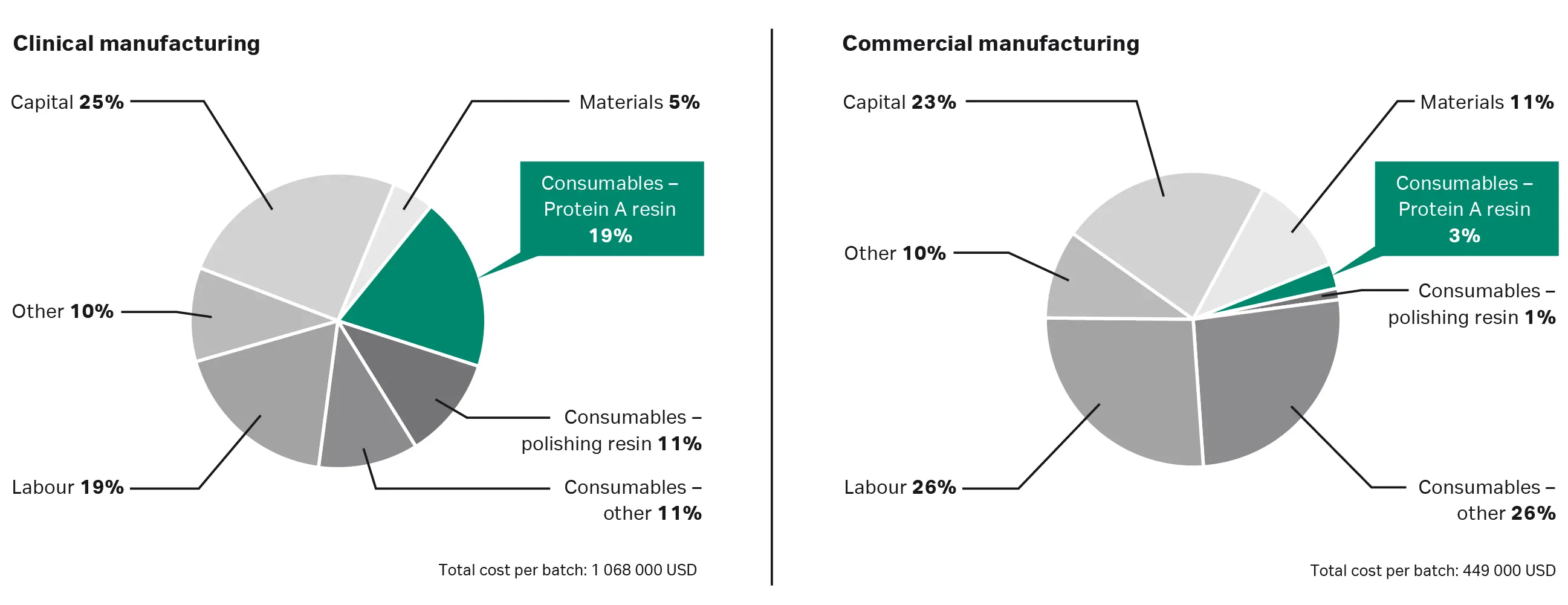
Figure 3 shows how this plays out in two different manufacturing scenarios. In a clinical scenario running four batches , protein A resin makes up 19% of the total cost, whereas it comprises only 3% of the cost of a commercial process using the resin for 200 cycles (Fig 3).
Fig 3. Comparing clinical and commercial manufacturing scenarios for a single-use process using protein A resin to purify 2000 L cultures at 3 g/L. In the clinical scenario, the resin’s full lifetime is not utilized, whereas in the commercial scenario it is.
In clinical manufacturing key milestones can make or break a drug development process. Thus, from the supplier side, expanding the resin toolbox to more accurately reflect the goals and constraints of these phases makes sense. And on the manufacturing side, choosing a resin that meets but doesn’t exceed your needs can improve cost and process efficiency without sacrificing product quality.
Balancing productivity and efficiency
Protein A resin cost per gram of mAb produced is just one way to measure productivity of the capture step. Volumetric productivity, based on grams of mAb produced per liter of resin per hour, is another way. Productivity can also be assessed in terms of total processing time or throughput (e.g., in hours per batch), including downtime between cycles or process steps. A longer process with lower throughput then would be less productive than a faster process with higher throughput.
Any of these metrics will still depend on the performance attributes of the capture resin, and there are three main characteristics to consider. The first (and possibly most obvious) is binding capacity. A high binding capacity reduces the number of cycles needed per batch and can also lower the column volume required. The second attribute is flow rate. Good flow properties allow for faster processing and provide flexibility in method design to optimize purification speed and reduce time in the suite. Finally, durability is essential. High durability ensures the resin can be used repeatedly and cleaned thoroughly, maintaining high binding capacity throughout its lifetime. This is particularly important in high utilization scenarios like commercial manufacturing. Below are some examples of these characteristics for selected Cytiva protein A resins (Table 2).
Table 2. Characteristics of MabSelect™ protein A resins used for calculations
| Resin | MabSelect PrismA™ resin Excellent productivity at commercial scale. Wide window of operation and well-suited for purification platforms. |
MabSelect PrismA™ X resin* Highest dynamic binding capacity (DBC) of all the MabSelect resins for cost-efficient mAb capture. Excellent durability. |
MabSelect™ SuRe 70 resin Cost-efficient resin at low utilization with Cytiva quality and support. |
| Particle (bead size) d50v (μm) | ~ 60 | ~ 50 | ~ 50 |
| Flow velocity (cm/h) 20 cm bed height |
300 | 220 | 220 |
| DBC (g/L resin)† |
74 | 82 | 75 |
| Alkaline stability (M NaOH) |
0.5–1.0 | 0.5–1.0 | 0.1–0.5 |
* Resin to be sales started during 2025
† Trastuzumab at 6 min residence time (RT) in HiScreen™ column
We have benchmarked our most recent protein A resins against the legacy MabSelect™ SuRe LX resin, figure 4 to 6 shows these process modeling examples.
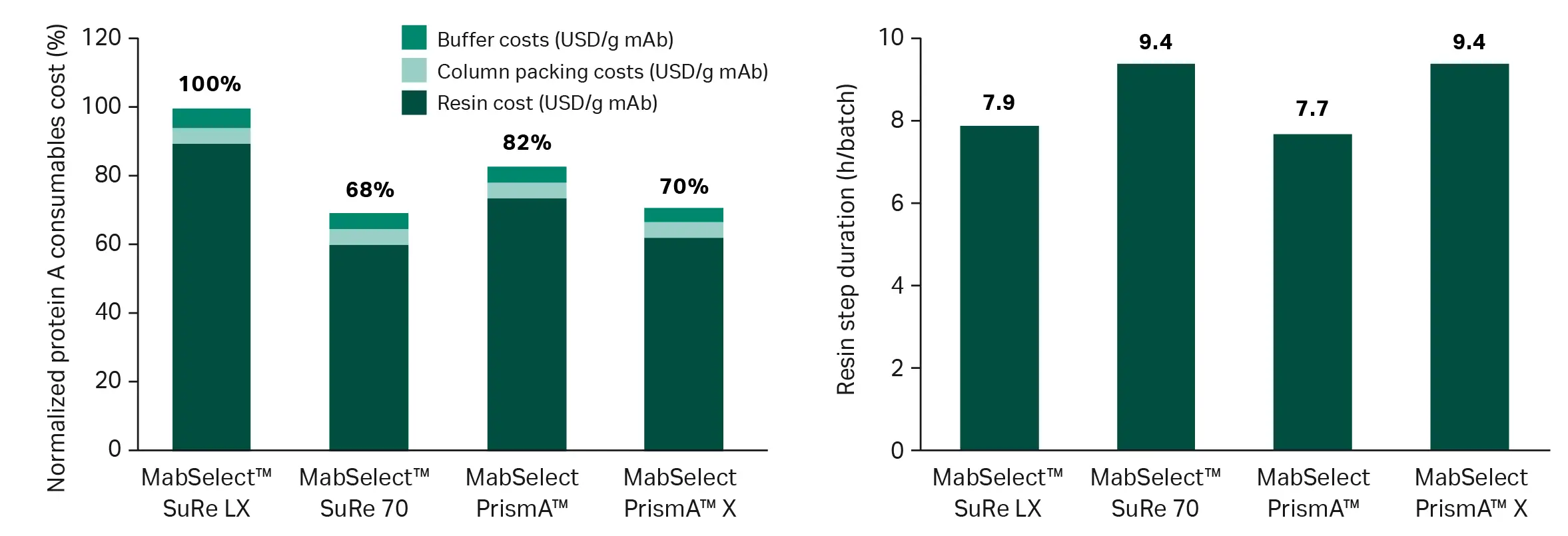
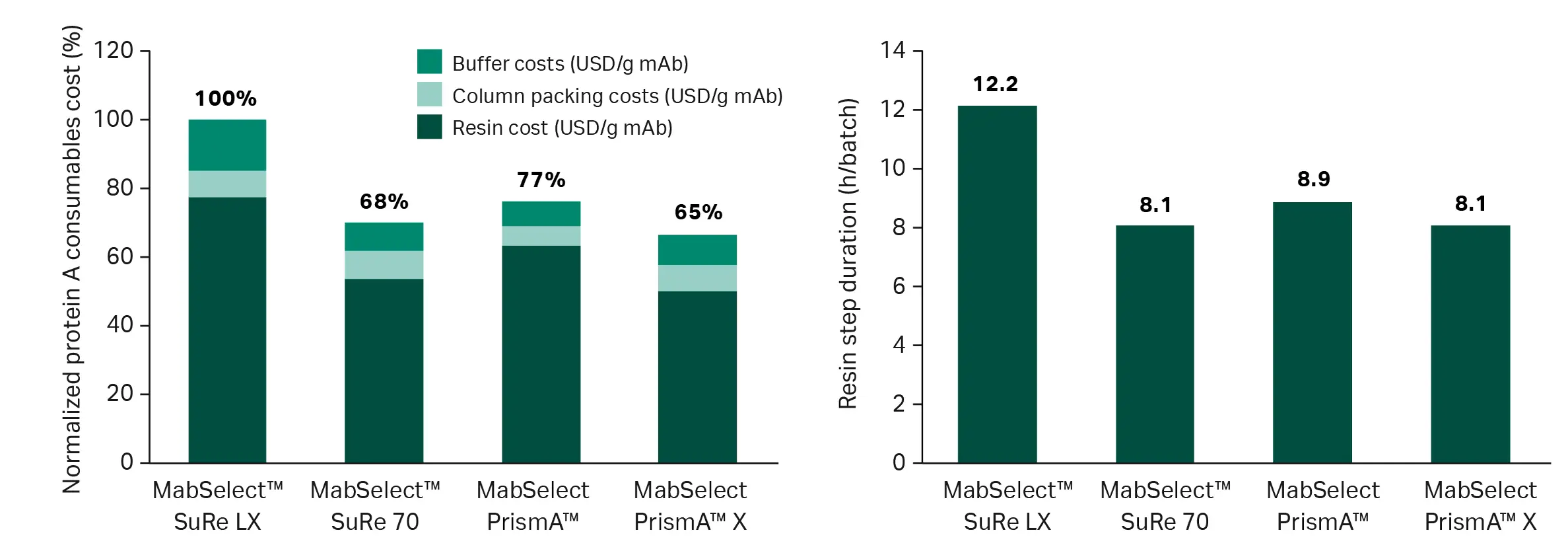
Figure 4 shows a typical clinical scenario modeled with four different Cytiva resins . MabSelect™ SuRe 70 and MabSelect PrismA™ X resins reduce consumable cost per gram of mAb by 30-32% compared to the legacy MabSelect™ SuRe LX resin. MabSelect PrismA™ resin lowers consumable costs more modestly, but, importantly, its flow properties also enable the shortest capture step.
Fig 4. Consumable cost per gram of mAb produced and step duration comparisons for a clinical process scenario. Input parameters are 3 batches from a 2000 L bioreactor volume, 5 g/L titer, 60 cm column diameter, 20 cm bed height, and a typical clinical pricing model. Note: The modeling uses fixed column diameters and residence times, meaning scenarios will be realistic but not fully optimized. Degree of optimization will vary substantially between resins, so outputs may not be fully representative.
While MabSelect™ SuRe 70 or MabSelect PrismA™ X resins may have slightly longer processing times, technologies like rapid cycling can help speed up production or reduce column volume by running higher flow rates and shorter residence times. Figure 5 shows the changes in relative consumable cost and step duration enabled by rapid cycling chromatography. The resin cost share decreases, while column packing and buffer costs increase slightly. This shift is due to reduced capacity utilization, which increases buffer consumption and repacking needs. However, in this scenario where the resin lifetime utilization is low, the total costs in rapid cycling mode are still lower than for the standard method.
Fig 5. Cost per gram of mAb produced and step duration comparisons for a clinical process scenario when rapid cycling chromatography is used. Input parameters are 3 batches from a 2000 L bioreactor volume, 5 g/L titer, 60 cm column diameter, 10 cm bed height, and a typical clinical pricing model. Note: The modeling uses fixed column diameters and residence times, meaning scenarios will be realistic but not fully optimized. Degree of optimization will vary substantially between resins, so outputs may not be fully representative.
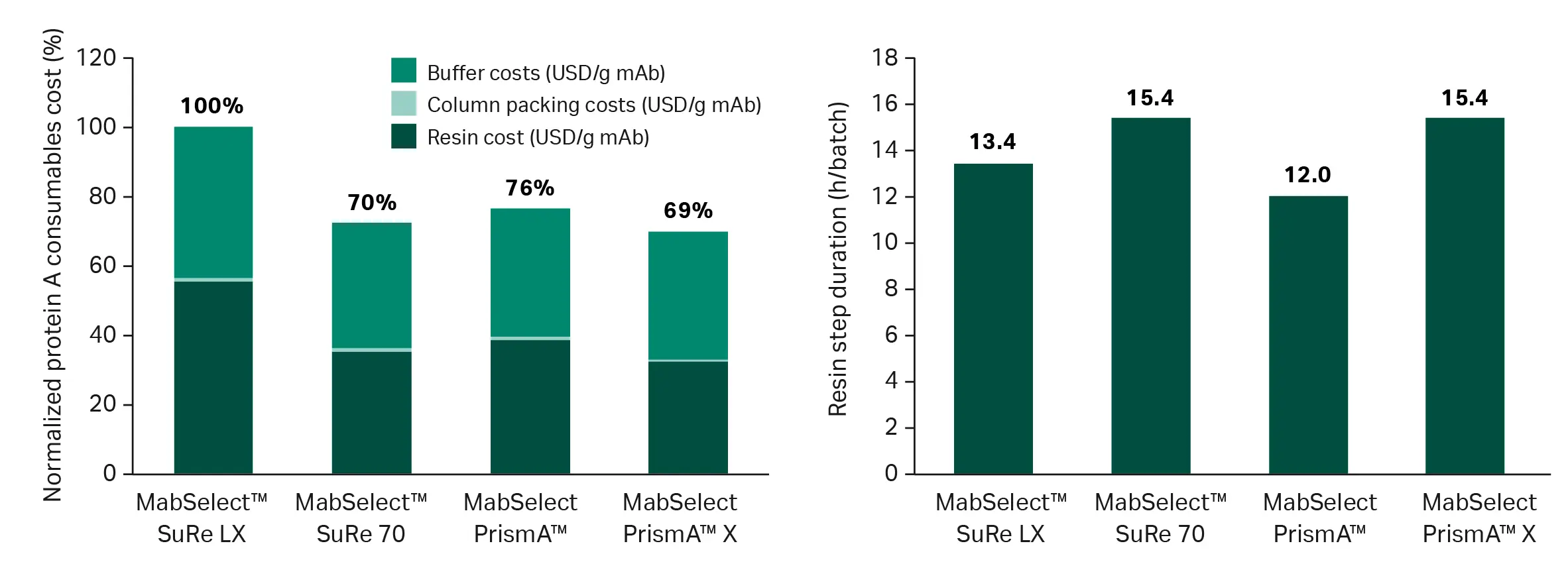
In a commercial manufacturing example with high titer and high volume (Fig 6), the capture resin makes up only about half the total consumable cost while buffer costs increase substantially, owing to improved resin lifetime utilization. In this scenario, where the resin’s full lifetime will be used, MabSelect™ SuRe 70 and MabSelect PrismA™ X resins still provide the lowest consumable cost per gram of mAb produced, but MabSelect PrismA™ resin's higher flow specifications provide the shortest total processing time, which may, in the total cost of goods calculation, have a much higher value than the reduced resin cost.
Fig 6. Cost per gram of mAb produced and step duration comparisons for a commercial manufacturing scenario. Input parameters: 15 000 L bioreactor volume, 10 g/L titer, 160 cm column diameter, and full resin utilization. Note: The modeling uses fixed column diameters and residence times, meaning scenarios will be realistic but not fully optimized. Degree of optimization will vary substantially between resins, so outputs may not be fully representative.
It takes more than specifications and productivity to select a resin
There is more to selecting a resin than technical specifications and productivity. Resins from Cytiva provide reliable and robust capture options across different phases. Our products have a track record of high quality and are well established in the industry. We have regulatory support around the resins and their use, an extensive security of supply program for timely availability of the products, and expertise and support for GMP manufacturing. Our resins are robust and scalable from lab to large-scale manufacturing, and we’re focused on environmental sustainability.
Summary
To summarize, input parameters influence both productivity and efficiency. Higher DBC improves productivity by reducing the number of cycles or column volume required. High flow rates improve productivity by reducing cycle time, and short bed heights can be an option when high back-pressure limits flow rates. Increased resin durability allows for use of more batches per resin lifetime. Good alkaline stability supports use of hundreds of cycles, exposure to strong cleaning agents with low loss of DBC, and excellent bioburden control.
Consider productivity, cost, and versatility to find the protein A resin that best matches your needs based on your process scenarios, volumes, development phase, and facility limitations. Think about your objectives and constraints and then evaluate to find the resin that best fits your needs. Process modeling is a useful tool to find the most optimal resin in regard to productivity and cost, but also consider other aspects such as quality, sustainability, and scalability.
At Cytiva, we can help you evaluate your clinical and commercial manufacturing needs and guide you in making informed decisions about which resins will best suit. Whether you’re aiming to optimize your process for cost-efficiency, productivity, or both, a helping hand is just around the corner.