Instrument qualification is more than a one-time service if you work in cGMP or GxP environments. Ensure ongoing regulatory compliance with qualification services at key points in your equipment lifecycle.
Qualification is more than a one-time event
If you’re working in a current good manufacturing practices (cGMP) or other current GxP environment, you know it’s critical to keep your equipment in working order. But what does that mean to regulatory authorities? It means that your equipment must be periodically evaluated and validated.
Because your equipment should always be tested in the exact same conditions as it is intended to be used, our instrument qualification services follow your complete equipment lifecycle. At some point in its lifetime your instrument may be repaired, relocated, upgraded, or modified in other ways. We can help you keep it qualified along the way .
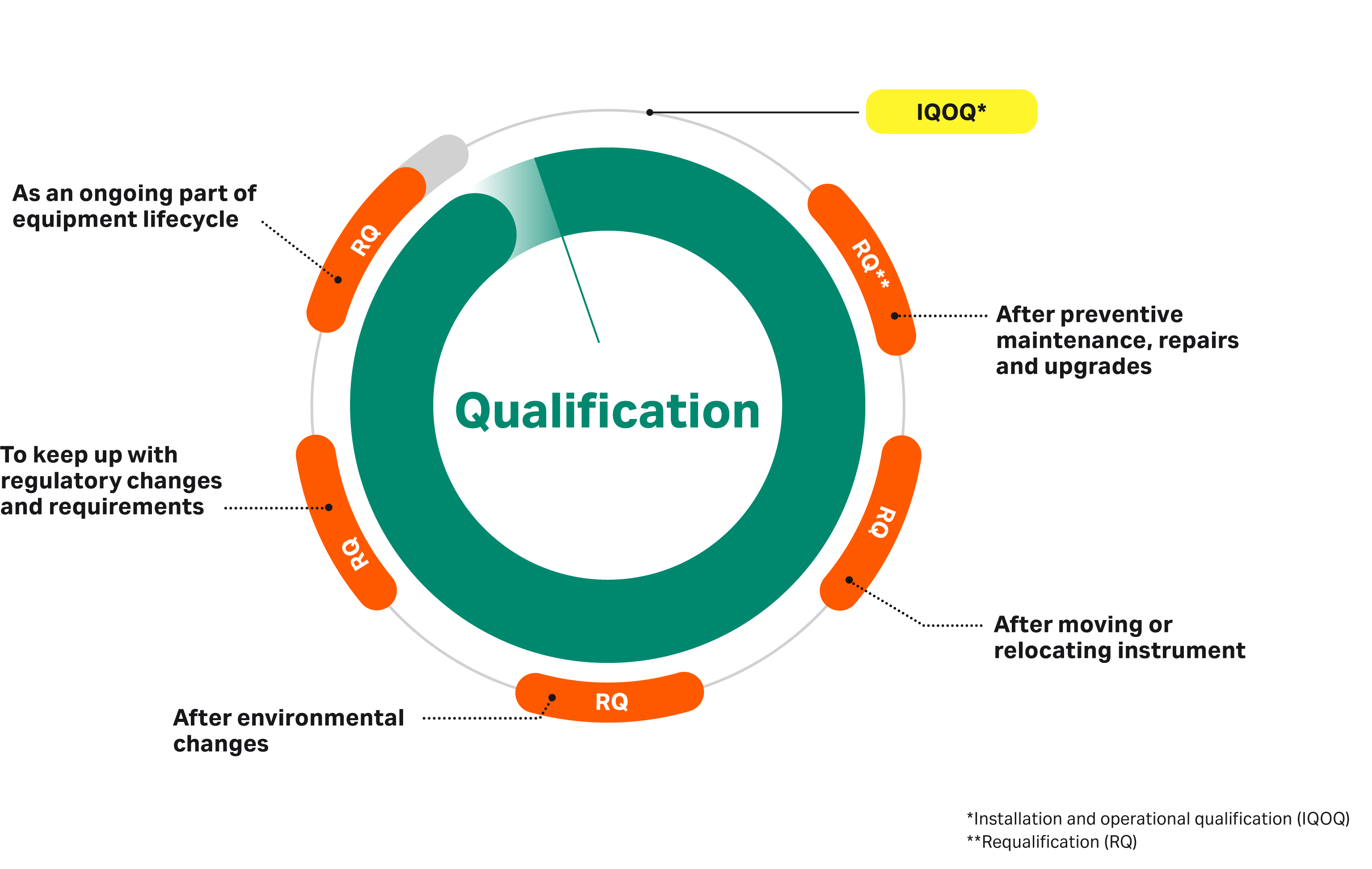
Qualification LifeCycle
What are the regulatory requirements for GxP?
Regulatory bodies are clear: it’s critical to properly maintain an instrument’s qualified state. Here are a few examples extracted from regional regulatory guidelines that emphasize this need – qualification is more than a one-time service at the time of installation:
- EU: “Equipment, facilities, utilities and systems should be evaluated at an appropriate frequency to confirm that they remain in a state of control.“ (1)
- Global (ICH): “Systems and processes should be periodically evaluated to verify that they are still operating in a valid manner.” (2)
- US: “The equipment and facility qualification data should be assessed periodically to determine whether re-qualification should be performed and the extent of that re-qualification. Maintenance and calibration frequency should be adjusted based on feedback from these activities.” (3)
- India: “Qualification and validation should not be considered a one-off exercise. An ongoing program should follow their first implementation and should be based on an annual review.” (4)
Five changes that might require requalification
- Environmental conditions have changed over time. Consider the impact of changes in temperature, humidity, or atmospheric pressure.
- Equipment has been moved/relocated. Manufacturing lines, electrical power, fixed pipe connections, and even environmental conditions can be dramatically different from room to room, building to building, or when relocating equipment to an entirely new facility.
- Preventive maintenance, repair, or upgrades have occurred. Replacing spare parts while the instrument is running could affect the specifications. Think about an event that replaces a major component on a system – affecting the wetted flow path and items in the electrical cabinet – or even pressurized air supply, if applicable. How can you confirm that replacing the part didn’t disturb any other functions of the instrument?
- Your needs have changed, which requires a modified binder. Projects and manufacturing needs change over time to meet new demands. The parameters, recording calibrations, and instructions must be updated according to the new utilization.
- Regulatory guidelines have changed. Maintaining compliance with regulatory bodies is a critical endeavor in GxP environments. Changes to regulatory guidelines require updated documentation.
Why does requalification matter?
Changes can affect your instrument’s performance and bring it to an unqualified state – creating risk for your operations:
- You could lose production batches if issues are discovered too late.
- You could waste time and money if you need to backtrack and launch investigations to discover deviation handling.
- You could fail to meet production or research deadlines.

Why select Cytiva for your qualification needs?
The OptiRun™ Service solutions team knows the instruments Cytiva designs and manufactures. We also understand how regulations affect the installation and use of our equipment. And we keep our knowledge up to date as regulatory guidelines change.
With our qualification services you’ll benefit from:
- Our access to R&D, and manufacturing, and engineers – they develop, produce, and maintain our instruments.
- The tools and software we use to develop and produce our qualification products, so you get full traceability and transparency.
- Our guidance when you’re considering an update to your instrument’s hardware or software. We’ll update our qualification documents if you proceed.
- Our global network of certified Qualification Services engineers, who will ensure timely and efficient qualification.
- Our efficient order-to-deliver process that we’ve fine-tuned. We manufactured more than 25 000 binders in four years.
- The flexibility to choose hard copy binders or eBinders.
- Updated test results as we compare performance post-qualification with original performance by our installation qualification (IQ) and operational qualification (OQ) service.
Learn more about qualification services
References
- EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Annex 15: Qualification and Validation. Ref. Ares(2015)1380025 - 30/03/2015.
- ICH Topic Q 7 Good Manufacturing Practice for Active Pharmaceutical Ingredients. November 2000 CPMP/ICH/4106/00.
- Guidance for Industry Process Validation: General Principles and Practices. U.S. Department of Health and Human Services Food and Drug Administration.
- The Central Drugs Standard Control Organization (CDSCO, India).
