Introduction on AAV vectors for gene therapy
Viral vectors are often used for gene transfer of specific tissue- or cell-type modifications. Several viruses have been investigated for use in cell and gene therapy; adeno-associated virus (AAV) has emerged as the main vector for gene therapies. Here we describe how we developed an efficient and scalable cell culture process for AAV production by evaluating and optimizing each process step.
Study overview – development of the viral vector production process
HEK293T cells were successfully adapted to suspension culture in a serum-free medium without animal-derived components. Triple-plasmid transfection using polyethylenimine (PEI) was optimized for different parameters and conditions using a design of experiments (DoE) strategy in shake flasks. The optimal concentration and ratio of plasmids, PEI, temperature, transfection volume, and incubation time were evaluated for transfection efficiency and virus productivity. We further developed conditions for production in single-use bioreactor systems.
Viral genomes (VG, full capsids) were determined by qPCR (VG/L), and total viral particle (VP) titer (VP/L) was determined by a commercial ELISA. We also developed a Biacore™ surface plasmon resonance-based method for total virus capsid titer quantitation. The percentage of full capsids was calculated as the ratio between viral genomes and viral capsids. A transduction assay based on flow cytometry was used to evaluate the infectious virus titer.
An optimized transfection protocol in shake flasks was generated based on the design of experiments (DoE) studies for AAV2, with titers above 1014 viral particles/L. The protocol has also been evaluated with other AAV serotypes, such as AAV5, AAV8, and AAV9, with similar productivities achieved. XDR-10 single-use bioreactor process was designed and optimized based on the shake flask results. Furthermore, a second process developed in WAVE™ bioreactors gave similar productivities. Thus, we developed a scalable, robust, and reproducible production of AAV from small-scale shake flasks up to 20 L in single-use bioreactor systems.
Upstream strategy for AAV production
Figure 1 illustrates the strategy to develop the upstream viral vector process.
Fig 1. The strategy was to develop a start-to-finish process for AAV production; here we present the upstream process development.
Materials and methods
For maintenance or expansion cultivation, we routinely passaged HEK293T adapted to suspension cells (CRL-3216 ATCC) at a VCD of 0.1 to 0.2 × 106 cells/mL. We kept cells in growth phase and not above 3 × 106 cells/mL to minimize cell aggregation in the culture, as we know this is an important consideration. Cells were counted using Vi-CELL™ (Beckman Coulter) and seeded in a new shake flask with HyClone™ brand HyCell™ TransFx-H medium (Cytiva). Medium was supplemented with 4 mM L-glutamine (Cytiva) and 0.1% Pluronic™ F-68 (ThermoFisher). We performed AAV production using Xcellerex™ XDR-10 or ReadyToProcess WAVE™ 25 (Cytiva) bioreactors.
Production of rAAV was achieved through transient transfection of HEK293T cells using a triple-plasmid system, which allowed production of functional AAV viral particles only in cells transfected with three plasmids, pAAVGFP Control Vector, pHelper Vector and pAAV-RC Vector (Cell Biolabs).
The transfection agent was polyethylenimine PEI MAX™, (Polysciences). Transfection efficiency and transduction assay, detecting GFP-expressing cells was evaluated by flow cytometry (BD Biosciences). Viral genomes (full capsids) were determined by qPCR (VG/L) and the total viral particle titer (VP/L) was determined by ELISA (Progen). The ratio of qPCR:ELISA was used to estimate the percentage of full capsids.
Results
Design of experiments
We used DoE methodology to optimize triple-plasmid transfection for rAVV production in an HEK293T suspension cell system. By using DoE strategy, we could evaluate the impact of 50 different conditions/parameters. The experiment was analyzed and evaluated by using MODDE™ (Pro Ver 12.0.1). To create a transfection protocol for the rAAV production process to generate high viral titer and percentage full capsids, the following parameters were investigated:
- Cell density
- Transfection volume (percent of total volume)
- Plasmid (DNA) concentration and ratio
- PEI/DNA ratio
- Incubation time of mix prior to transfection
- Temperature
- Supplement
- Time of harvest (TOH)/post-transfection
Results are presented in Figures 2–8.
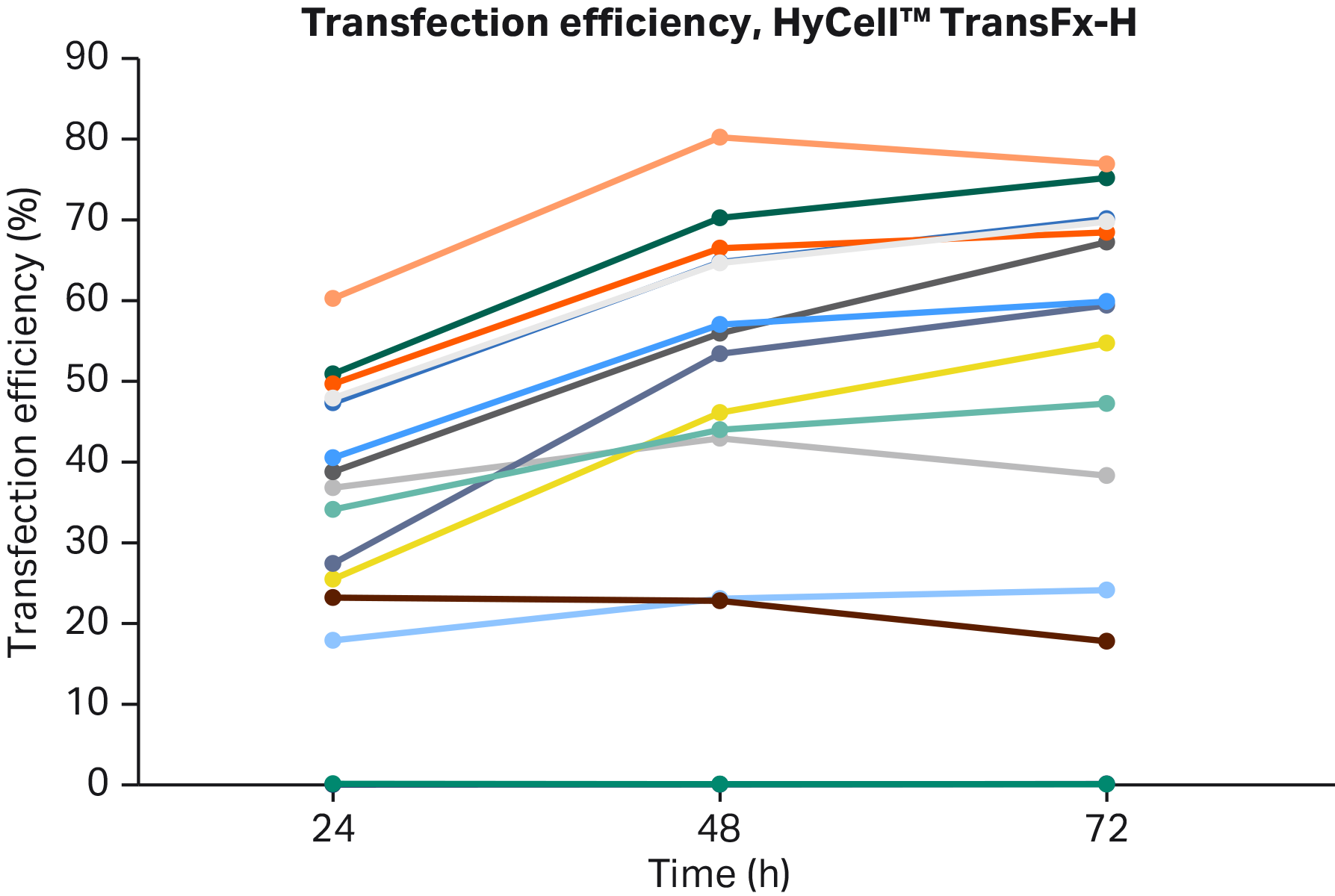
Fig 2. AAV vector transfection efficiency. Large variation between different conditions could be seen as percent of GFP positive cells.
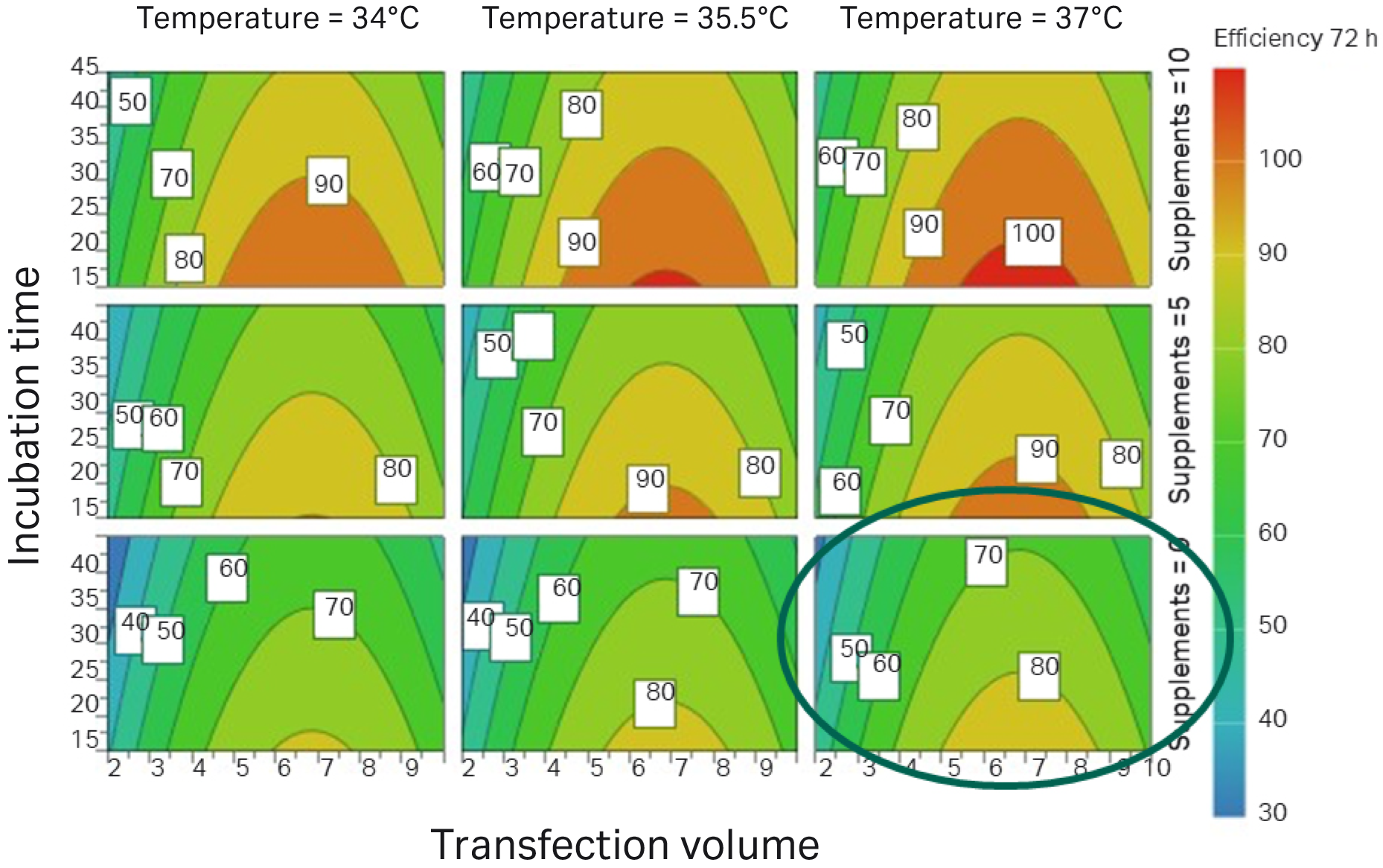
Fig 3. Transfection volume (percentage of total volume). By adding supplements, transfection efficiency was improved compared to previous DoE (marked by a circle).
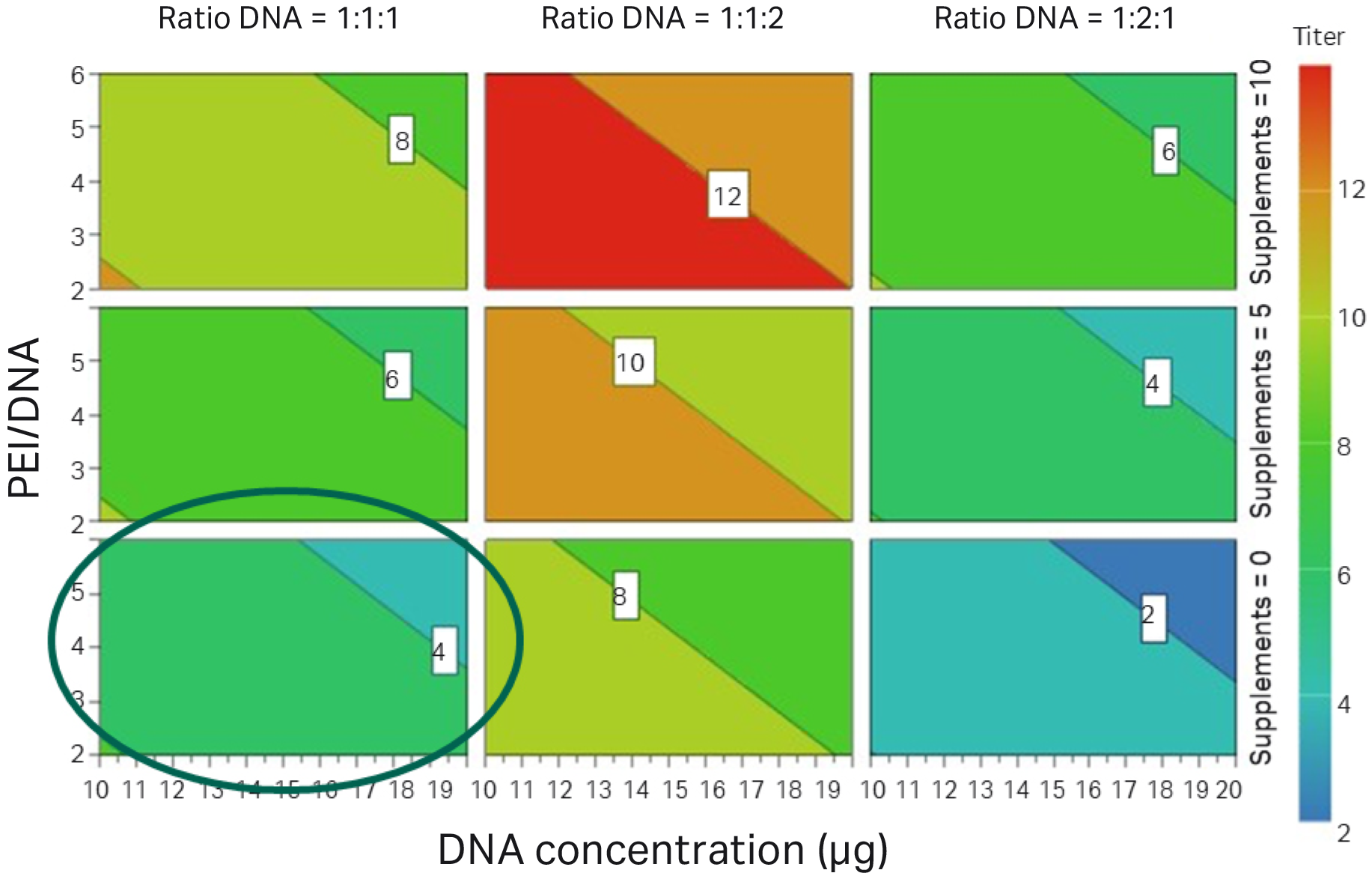
Fig 4. DNA ratio (Rep/cap: helper: transgene GFP) 1:1:2 and addition of supplements improved functional titers compared to previous DoE (marked by a circle).
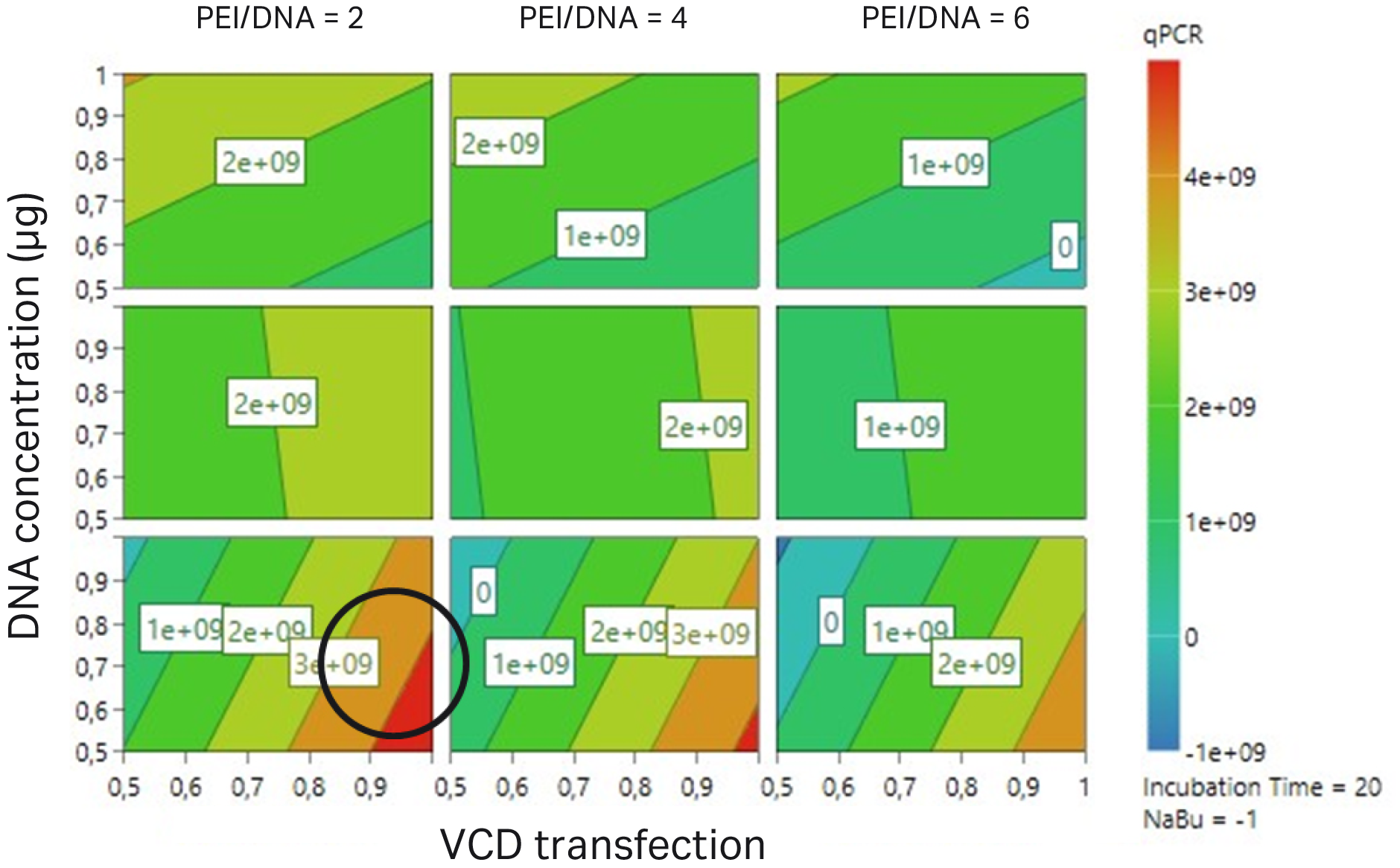
Fig 5. The circle indicates that improvement could be achieved when increasing VCD and a ratio of 2:1 of PEI/DNA. DoE study analyzed with qPCR method to confirm viral genome titer for different conditions.
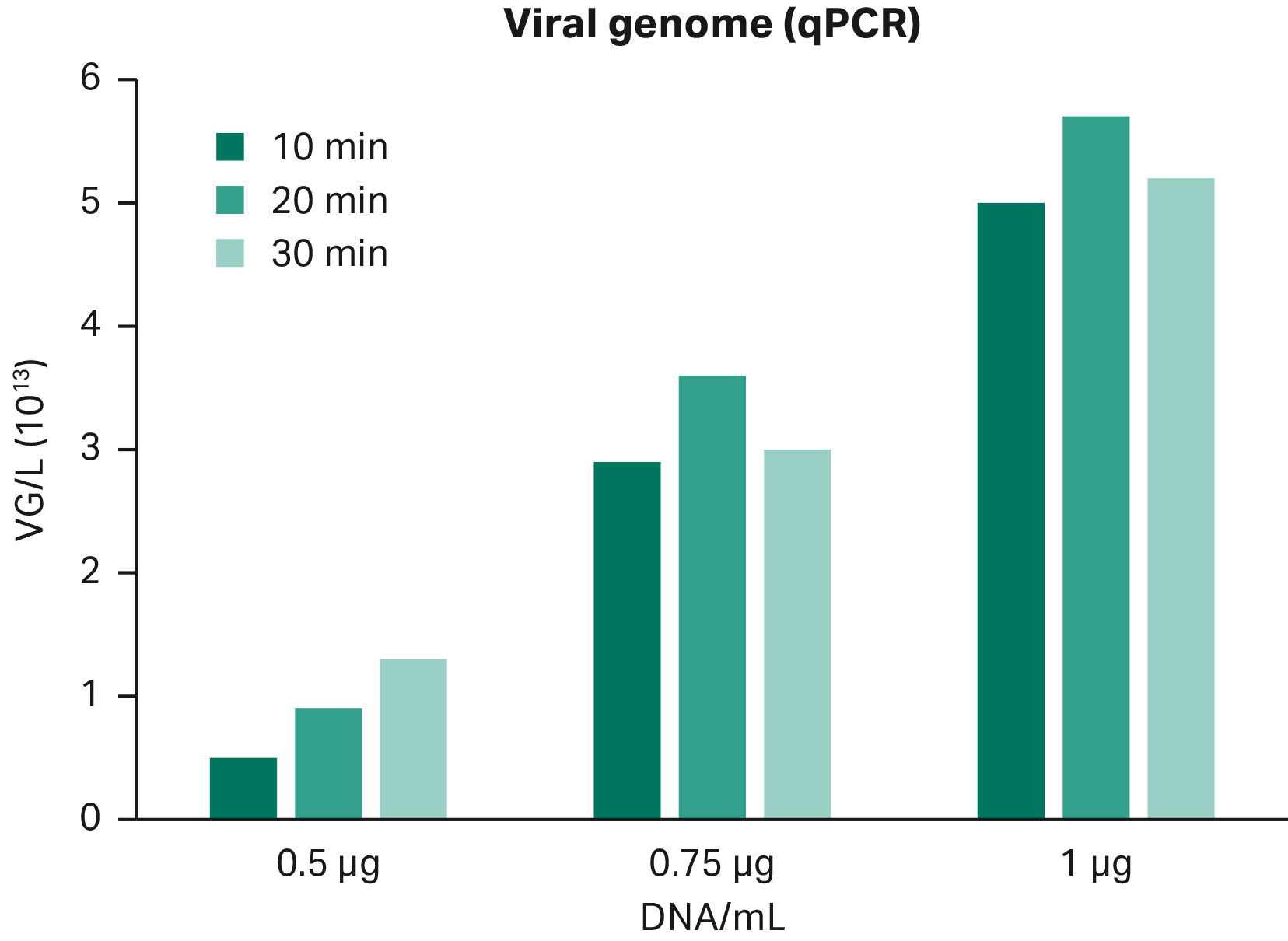
Fig 6. Increasing DNA concentration gives higher viral genome titer. Transfection incubation time has a very small impact on the viral genome response.
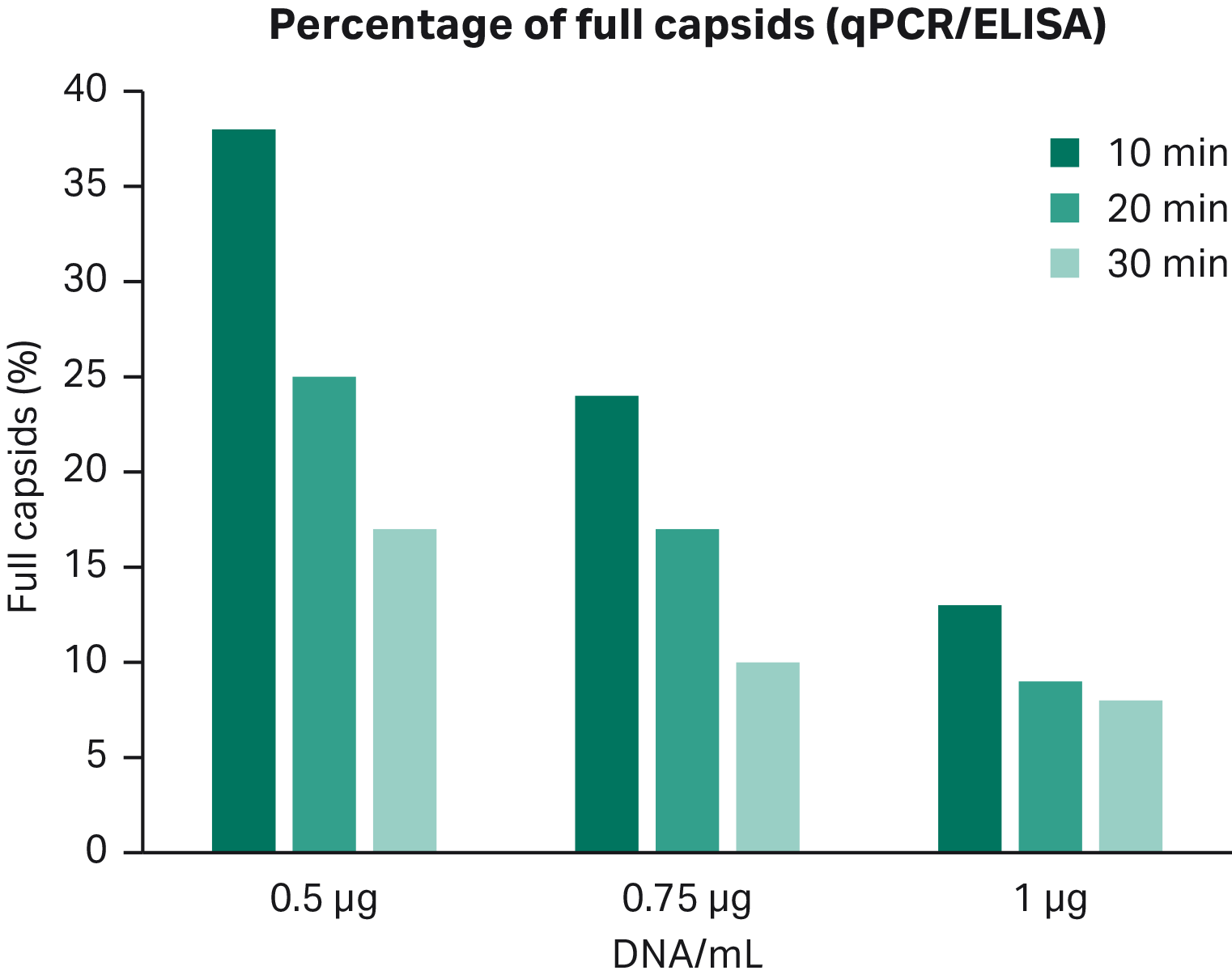
Fig 7. Increased DNA concentration gives lower percentage of full capsids. Transfection incubation time affects the percentage of full capsids more when using low concentration of DNA.
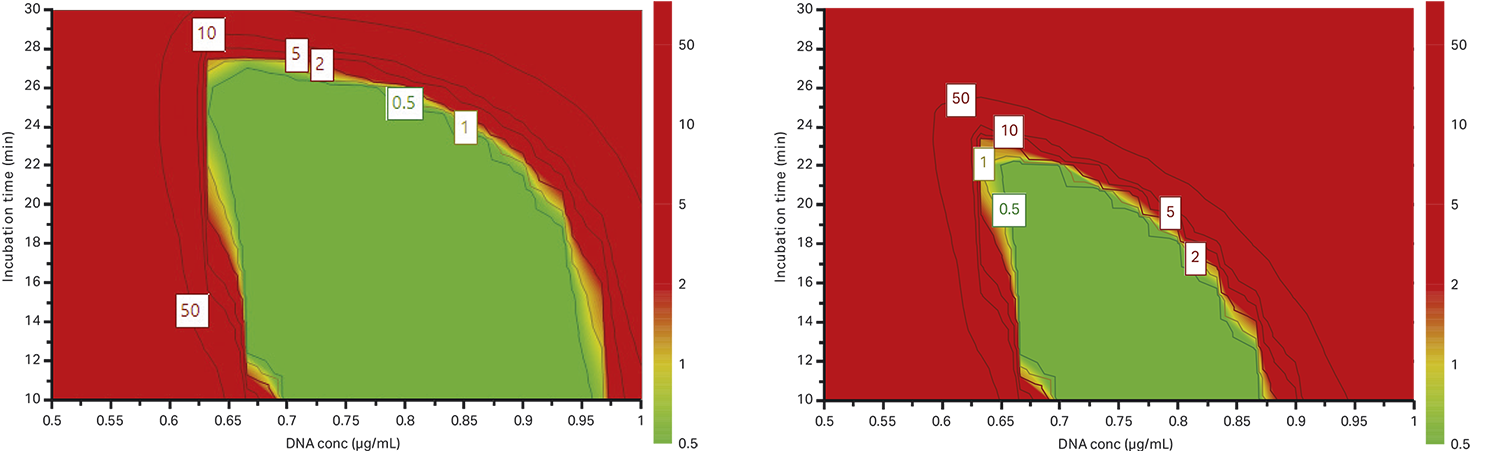
Fig 8. More realistic scenario using design-space optimization, where we investigate the probability of failure to reach (A) 10% full capsids and (B) 15% full capsids. Decreased transfection time and maintained DNA concentration, improvement could be seen based on percentage of full capsids.
Scalability and production
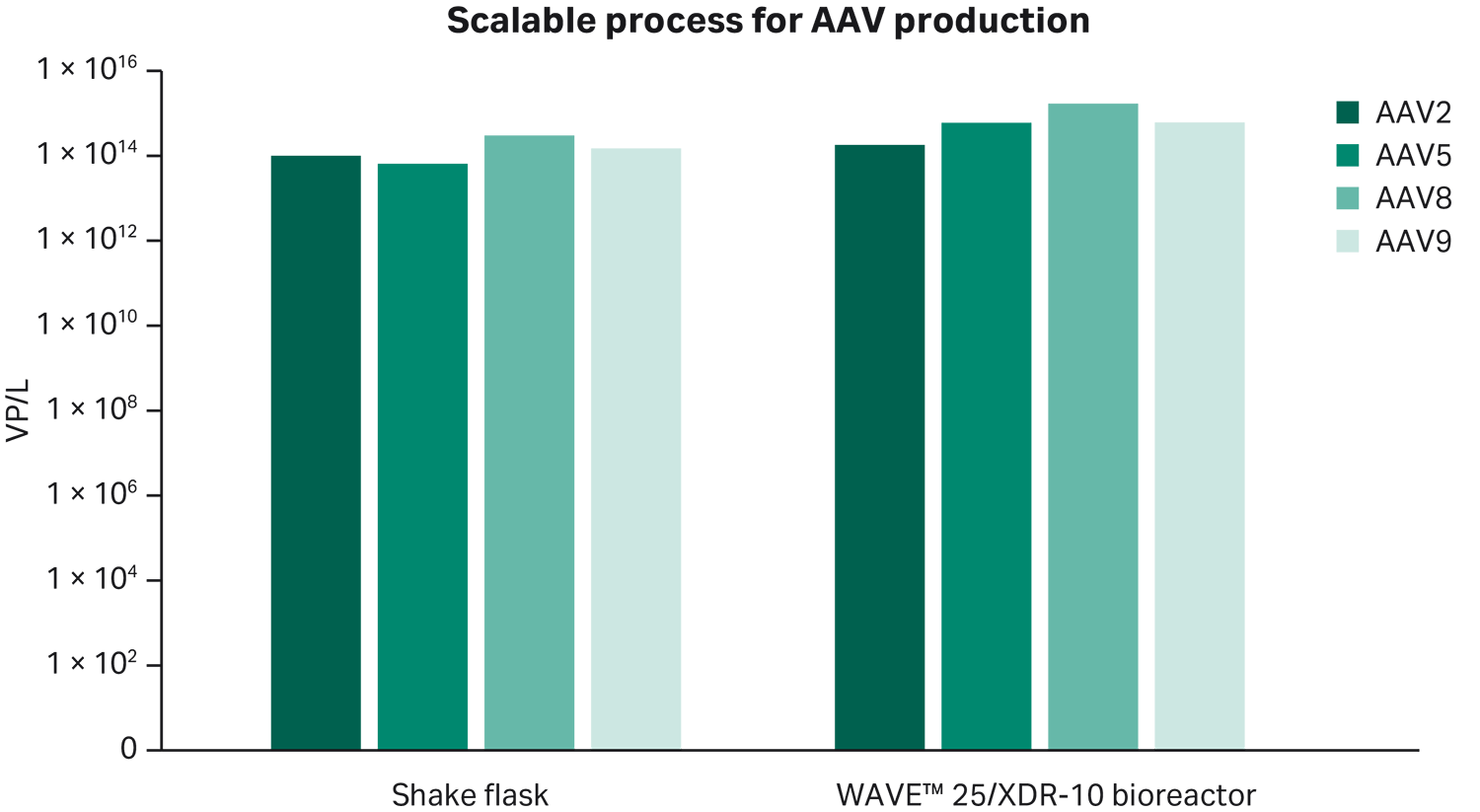
Results of all scales, up to 25 L, are presented in Figure 9.
Fig 9. Successful scalability up to 25 L for several AAV serotypes.
Final transfection protocol for rAAV production
- VCD: 1 × 106/mL
- DNA (μg/mL): 0.75
- PEI/DNA ratio: 2:1
- Transfection volume (percent of total): 5
- Incubation time: 15 min, RT
- DNA ratio: 1:1:2 (Rep/cap: helper: transgene GFP)
Conclusions
The aim of this study was to create a production process for recombinant AAV vectors, generating both high viral titers and high percentage full capsids in a scalable, robust process. The developed transfection protocol can be used for several AAV serotypes, such as AAV2, AAV5, AAV8, and AAV9. To develop a full process "start-to-finish", we used rAAV5 as a process model and developed a protocol.
The following titers were achieved in the harvest material to produce rAAV5:
- ELISA: ≥ 1 × 1014 VP/L, qPCR: ≥ 1 × 1013 VG/L, and ≥ 15% full capsids
- The percentage of full particles decreased with higher DNA concentration, which indicated that the optimal point for both high viral titer and percentage full capsids was achieved by transfecting using 0.75 μg of DNA/mL
- Decreasing the transfection incubation time appears to increase the percentage full capsids
Learn more about solutions to make AAV viral vectors for gene therapy.