Cell freezing by cooling at a controlled rate
Successful cryopreservation of cells requires a controlled cooling rate. Cooling too rapidly or too slowly could lead to a poorer outcome.
A controlled cooling rate is needed because of the physical environment experienced by the cells during cryopreservation. As ice forms in the extracellular space, pure water is locked away as ice. This results in cells being suspended in an increasingly concentrated solution, which dehydrates cells. However, cooling too slowly makes this solution toxic. And cooling too rapidly prevents cells from dehydrating sufficiently; intracellular ice forms.
Controlled-rate cooling is achieved using equipment such as VIA Freeze controlled-rate freezers (Fig 1).
Fig 1. VIA Freeze controlled-rate freezer.
An important protocol parameter to understand is the temperature at which it is safe to stop the controlled cooling protocol and transfer cells to long-term storage, typically liquid nitrogen storage. Surprisingly, there have been few studies to define the endpoint at which cells can be transferred to storage conditions. Temperatures from -35°C to -130°C are in use.
This study explored the critical temperature for the endpoint of the controlled cooling phase. Defining that value would minimize the amount of time samples must be cooled in a controlled-rate freezer. By reducing cooling time per run, there is the potential to increase the number of runs per day.
Design of cooling endpoint study
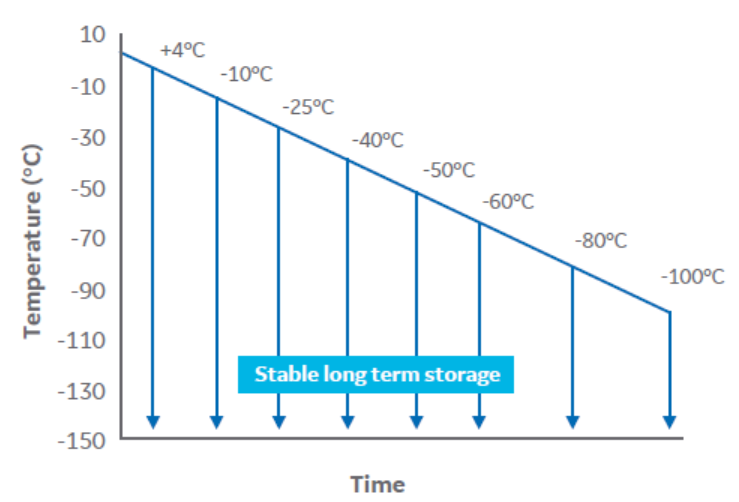
To determine the biological implications of the controlled cooling endpoint temperature, cell samples in cryovials (1 mL fill volume) were cooled at 1°C/min in a VIA Freeze system (Cytiva) over a range of temperatures from 4°C to -100°C (Fig 2).
Fig 2. A schematic of the experimental design.
At each selected temperature, sets of 5 samples were plunged into liquid nitrogen. The cryovials were stored below -140°C for at least 24 h, and then thawed.
Jurkat cells (human suspension immune) and Chinese hamster ovary (CHO) cells were evaluated. Dimethyl sulfoxide (DMSO, 10%) was used as cryoprotectant. After thawing, cells at each endpoint were grown in cell culture medium under standard conditions. Viable cell counts were determined after 24, 48, and 72 h of growth. A Cytell Cell Imaging System (Cytiva) was used for analysis.
Results
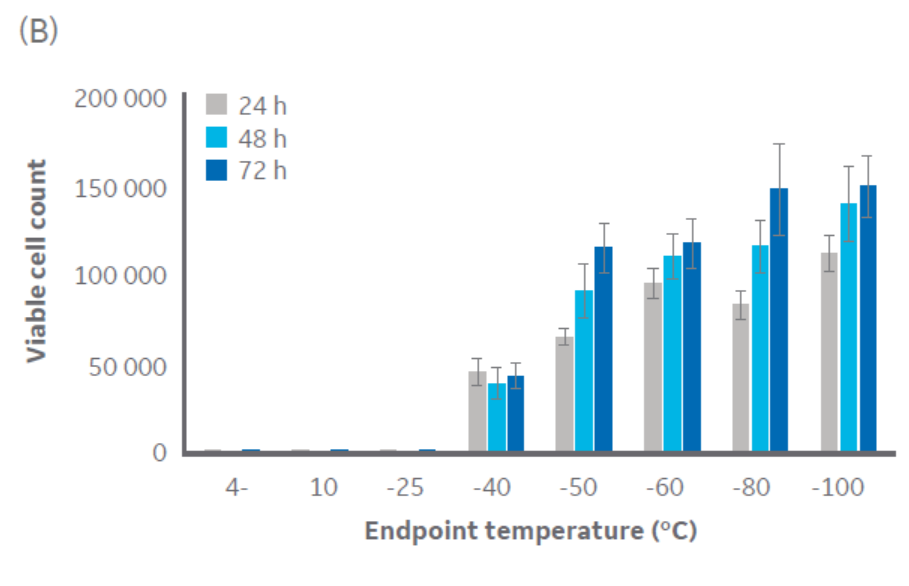
According to Figure 3, temperatures at or below approximately -40°C are sufficient for CHO cell cryopreservation. Similar results were obtained with Jurkat cells (Fig 3), with a critical temperature between -40°C and -50°C. The same pattern was observed with HepG2 and MG-63 cell lines (data not shown, to keep the article brief).
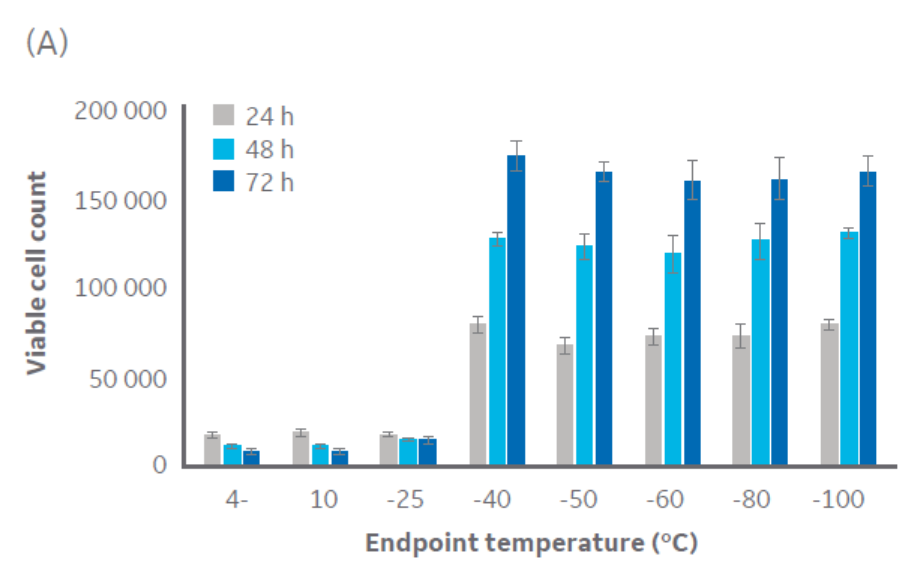
Fig 3. Viable cell count for (A) CHO cells and (B) Jurkat cells at 24, 48, and 72 h of culture post-thaw with different endpoints during controlled-rate cooling. n = 5 ± SD.
Conclusions
This study demonstrates that the key temperature during linear cooling is between -40°C and -50°C, and further cooling offers no additional advantages. Physically, this finding can be attributed to an intracellular colloidal glass transition at -47°C. At this temperature the intracellular compartment solidifies, and the cells cannot respond osmotically. In addition, there is no free water to form intracellular ice. Thus, the key factors for cryopreservation-induced cell death are eliminated.
Advantages of choosing a higher endpoint
Compared with cooling to -100°C, for example, a higher endpoint can be chosen. This means that cycle times can be shorter for cryopreservation protocols, allowing multiple runs in a day. A temperature such as -60°C is recommended to give a safety margin in case samples warm during transfer to long-term storage.
Because the maximum cooling rates VIA Freeze systems can achieve is temperature dependent, larger volumes can be processed when choosing -60°C as an endpoint over -80°C or -100°C.
Read article on controlled-rate freezing of cord blood cells.
Learn about Cytiva solutions for cryogenic cold chain management.