Performing dilution, concentration, and washing steps in a closed and automated manner is important in GMP cell therapy manufacturing. To assist in this process, Cytiva offers the CultureWash C-Pro application — a flexible, closed, and automated cell washing procedure — for use on the Sepax C-Pro cell processing system. Using a model T-cell line, we demonstrate the impact of varying rinse volume, intermediate volume, and manual extraction parameters on overall cell recovery with a final product dose volume of 20 mL. In this study, > 86% recovery was achieved, with 95% average recovery when manual extraction was enabled. We also investigate compartments where cell loss occurred and discuss strategies to maximize cell recovery.
Introduction: CultureWash C-Pro application
The closed and automated Sepax C-Pro cell processing system allows for flexible automation of multiple steps in the cell therapy workflow. The instrument, along with applicable software applications and single-use disposable kits, can be used for upstream concentration, purification, isolation, and viral transduction as well as downstream harvesting, washing, resuspension, and formulation of cells for cryopreservation.
This application note specifically covers recommendations for the CultureWash C-Pro application, where the final volume is low (20 mL) and with a starting cell number of 1 ×1010 cells. CultureWash C-Pro is a flexible application for dilution, concentration, and washing of fresh or frozen cell products. Figure 1 outlines where CultureWash C-Pro can be used in the T-cell workflow.
Fig 1. General workflow for cell therapy manufacturing. The CultureWash C-Pro application on the Sepax C-Pro cell processing system offers solutions for the steps highlighted in green (or green arrows if used between unit operations).
There are two single-use kits — CT-60.1 and CT-90.1 (Fig 2.) — that can be used with the CultureWash C-Pro application. Both kits contain a separation chamber, pressure monitor, stopcock manifold, initial line, washing solution line, 1 L waste bag, and final line. The manual extraction option can only be enabled when using the CT-90.1 kit, as this kit has an additional line on the center of the stopcock (labeled “Final ME” in Figure 2) used to manually extract cells into the attached vessel.
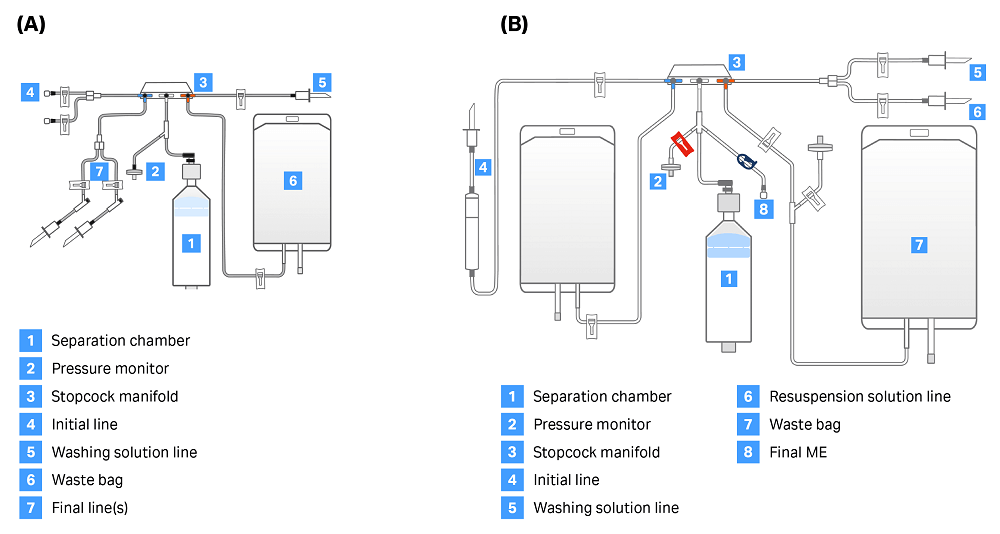
Fig 2. Sepax C-Pro kit options for CultureWash C-Pro application. (A) CT-60.1 kit, used for automatic extraction. (B) CT-90.1 kit, used when enabling manual extraction. The line marked “Final ME” is necessary to enable removal of cell solution from the line between the air line and the chamber.
The aims of this study were to:
- Investigate average recovery expected with 10 billion total cells and 20 mL final volume
- Explore the effect of rinse volume on cell recovery
- Maximize cell recovery using the manual extraction option
Materials and methods
Cell culture
We thawed and cultured Jurkat cells (ATCC, Clone E6-1) in Nunc™ treated T225 flasks (Thermo Scientific) in RPMI 1640 (Cytiva) with 10% fetal bovine serum (FBS, Cytiva), 1% SG-200 (Cytiva), 4.5 g/L glucose (Gibco), 100 units/mL penicillin-streptomycin (Cytiva), and 0.1% Pluronic (Gibco). Moving forward, we will refer to this media formulation as “complete medium”.
After 2 days in culture, cells were seeded in 650 mL complete medium at 4.5 × 105 cells/mL in a modified 10 L Xuri Cell Expansion System Cellbag bioreactor with perfusion, pH, and dissolved oxygen (DO) sensors (Cytiva) on a Xuri Cell Expansion System W25 (Cytiva). Cells were counted daily with complete medium added to maintain ~5 × 105 cells/mL until the volume in the Cellbag bioreactor reached the working volume of 5 L. We began perfusion of 2.5 L complete medium/day on the fifth day of Xuri culture, and perfusion rate was increased over time to control lactate, ammonium, and glucose levels, measured on a metabolite analyzer (see “Analytics” section). UNICORN software (version 7.0.2), the DO sensors on the Cellbag bioreactor, and increasing rock rate maintained DO above 80%. Starting on the eleventh day of Xuri culture, we removed cells daily for Sepax system runs.
Sepax C-Pro processing
Each processing run was performed on a Sepax C-Pro system with 1 × 1010 cells using CultureWash C-Pro v432 and with PLASMA-LYTE A (Baxter) with 10% human serum albumin (Gemini Bio), and 1 mM EDTA (Invitrogen) as the wash buffer. We kept operating parameters consistent across all runs unless a parameter was varied based on the condition being tested (see Table 1). Input volume varied from 725 to 936 mL, depending on the cell concentration in the Xuri culture at the time of cell removal. Intermediate volume varied from 7.5 to 20 mL to enable testing of a broad range of intermediate and rinse volumes. Users cannot input rinse volume in the CultureWash C-Pro application. In the absence of manual extraction, rinse volume is calculated as:
Rinse volume = Final volume - Intermediate volume
Thus, the rinse volume in this study varied from 0 to 12.5 mL, depending on the tested conditions.
Manual extraction was only enabled for condition IV10ME, where manual extraction was being tested (Table 2). Because manual extraction is not an option with the CT-60.1 kit, the IV10 condition required the use of the CT-90.1 kit, modified to remove the bubble chamber in the input line. For all runs, we used a 100 mL Labtainer™ bioprocess container (Thermo Scientific) as the final bag and a tube stripper to strip lines post-processing. See Table 2 for a complete summary of differences in each tested condition.
Table 1. Sepax C-Pro parameters: parameters that varied across conditions in bold
| Sepax C-Pro parameters | Units | Value |
|---|---|---|
| Protocol | CultureWash C-Pro v432 | |
| Total cells | cells | 1 × 1010 |
| Initial volume | mL | 725 – 936 |
| Detect initial volume | No (0) Yes (1) | 0 |
| Dilution ratio | 0 | |
| Dilution speed | mL/min | NA |
| 60s-mixing after dilution | No (0) Yes (1) | NA |
| Thaw bag validation | No (0) Yes (1) | 0 |
| Hang bag validation |
No (0) Yes (1) |
NA |
| Input bag rinsing | No (0) Yes (1) | 1 |
| Input bag rinse volume | mL | 50 |
| Pause input bag rinse | No (0) Yes (1) | NA |
| Optical cell detection | No (0) Yes (1) | 0 |
| Product filing speed | mL/min | 120 |
| Waste extraction speed | mL/min | 120 |
| Intermediate volume | mL | 7.5 – 20 |
| Pre-wash cycles | cycles | 1 |
| Pre-wash g-force | × g | 400 |
| Pre-wash sedimentation time | seconds | 300 |
| Switch washing solution | No (0) Yes (1) | 0 |
| Post-wash cycles | cycles | 0 |
| Post-wash g-force | × g | NA |
| Post-wash sedimentation time | seconds | NA |
| Switch resuspension solution | No (0) Yes (1) | 0 |
| Exchange waste bags | No (0) Yes (1) | 1 |
| Final volume | mL | 20 |
| Manual extraction | No (0) Yes (1) | 0 or 1 |
| Syringe for manual extraction | No (0) Yes (1) | 0 |
Table 2. Summary of differences in each Sepax condition tested
| Condition | Intermediate volume (mL) | Rinse volume (mL) | Manual extraction | Kit |
|---|---|---|---|---|
| IV7.5 | 7.5 | 12.5 | No | CT-60.1 |
| IV10 | 10 | 10 | No | CT-60.1 |
| IV20 | 20 | 0 | No | CT-60.1 |
| IV10ME | 10 | 0 | Yes | CT-90.1 |
Sepax C-Pro sampling
Samples were taken from each compartment of the Sepax C-Pro kit for each run, and cell counts were performed in triplicate to estimate cell loss and recovery. To estimate volume, we weighed each bag empty and full. Sampling of the initial bag enabled calculation of total input cells. To allow for separate cell counts for waste after concentration and waste after wash, the waste bag was replaced after the concentration step.
After the run, the initial bag was rinsed with a measured volume of media. We then determined cell concentration to estimate cells left over in the input bag. Because the concentration of cells was below the threshold for cell counts in the waste after concentration step, ~50 mL of waste after concentration was centrifuged at 400 × g for 10 minutes. The supernatant was aspirated, and the cell pellet was resuspended in ~1 mL media remaining in the tube. Cells were counted in triplicate and total cells in the 1 mL sample were multiplied by total sample volumes in the bag to estimate cells in the waste bag. To estimate cells left in the dead volume, air was pumped through the pressure sensor filter to move the chamber piston downward. The pressure sensor line was then sealed, and the stopcocks were positioned to direct remaining cells from the chamber into the lower left line. We then used the manual purge tool to move the piston, displacing the solution in the dead volume into a weighed conical tube. Please see Figure 3 for a summary of sampling procedures.
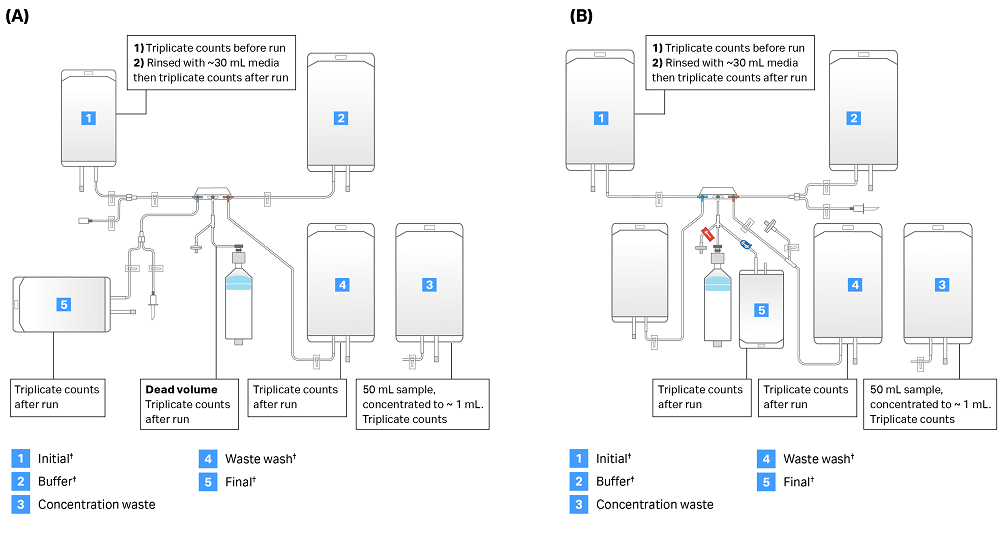
Fig 3. Sepax C-Pro sampling summary. Identical samples were taken from each Sepax C-Pro run. Bag locations and sampling procedures are shown for (A) CT-60.1 kit and (B) CT-90.1 kit. Note: Kits are shown in their configuration at the end of each run. Users must supply bags denoted with a dagger (†) as they are not part of the kit.
Analytics
We used a BioProfile™ Flex 2 Automated Cell Culture Analyzer (Nova Biomedical) to monitor the Xuri culture’s chemistry and gas. Cellbag sensors and UNICORN software version 7.0.2 (Cytiva) monitored pH and dissolved oxygen. All cell counts were performed in triplicate using a Via1-Cassette™ device on a NucleoCounter™ NC-200 Automated Cell Counter (Chemometec). Samples were diluted in media as was appropriate prior to cell count. We estimated volumes in bags and tubes by subtracting the empty vessel weight from the full vessel weight.
Statistics
We determined statistical significance in GraphPad Prism 8 using an ordinary one-way ANOVA and Tukey’s multiple comparison test with a single pooled variance. Significance is denoted in each graph as * for p < 0.05, ** for p < 0.01, *** for p < 0.0001.
Results and discussion
Cell recovery and viability
Average recovery for all conditions was greater than 86% (Fig 4). Recovery was higher, on average, for runs with manual extraction (IV10ME = 95.7% ± 7.3%) and similar for all conditions where manual extraction was not used (IV7.5 = 86.1% ± 3.4%, IV10 = 88.6% ± 0.5%, IV20 = 86.7% ± 2.8%). Differences in recovery across groups were not statistically significant.
The difference in pre- and post-processing cell viability was consistently less than 1% for each run across all conditions, with post-processing viability > 98% for all conditions (data not shown).
These data indicate that CultureWash C-Pro on the Sepax C-Pro system can achieve high recovery, even with low final volumes and a high final cell concentration. For low final volumes, using a manual extraction method might increase cell recovery.
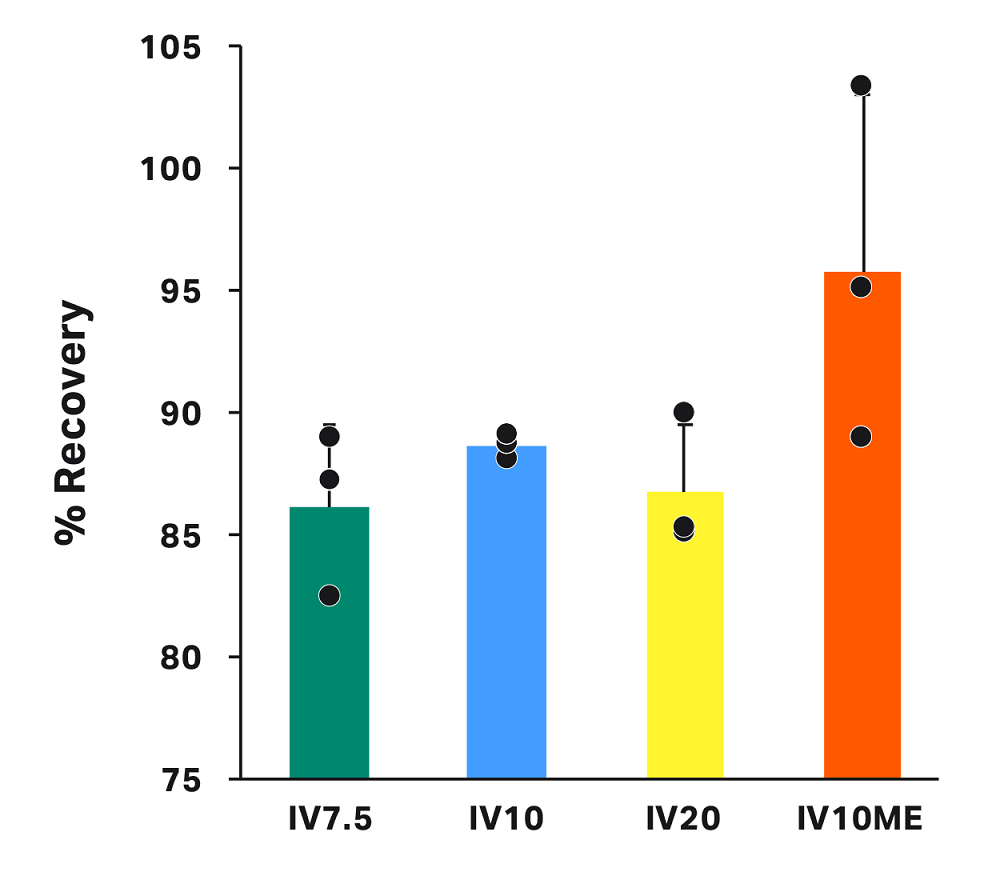
Fig 4. Recovery in the final bag for each condition. Each bar represents the average of technical triplicates ± SD, with each replicate shown in black. Differences are not statistically significant.
Total cell loss
To explore the compartments of cell loss in each condition, we retrieved samples from each compartment of the kit — as outlined above under “Sepax C-Pro sampling" — and calculated percentage of total cells per compartment (Fig 5).
Percentage of cells left in the initial bag was variable, but less than 1.5% of total cells were left in the initial bag across all conditions. A 50 mL bag rinse was included in these runs. You can minimize cells left in the initial bag by ensuring the bag is positioned in a way that the entire cell solution can enter the centrifuge chamber and by including an initial bag rinse, up to 100 mL, in the protocol settings.
Cells in the waste after concentration step were minimal (< 0.3% for all conditions).
Cells left in dead volume and cells in the waste after wash are both affected by the test conditions, discussed in the next sections.
The remainder of the loss comes from the calculated mass balance. This is the percent of total cells that were not accounted for in the tested Sepax compartments. Some cells might remain in the Sepax lines and centrifuge chamber. The mass balance could also reflect the inherent error in the volume estimation and cell counts, due to the large dilutions required for some samples.
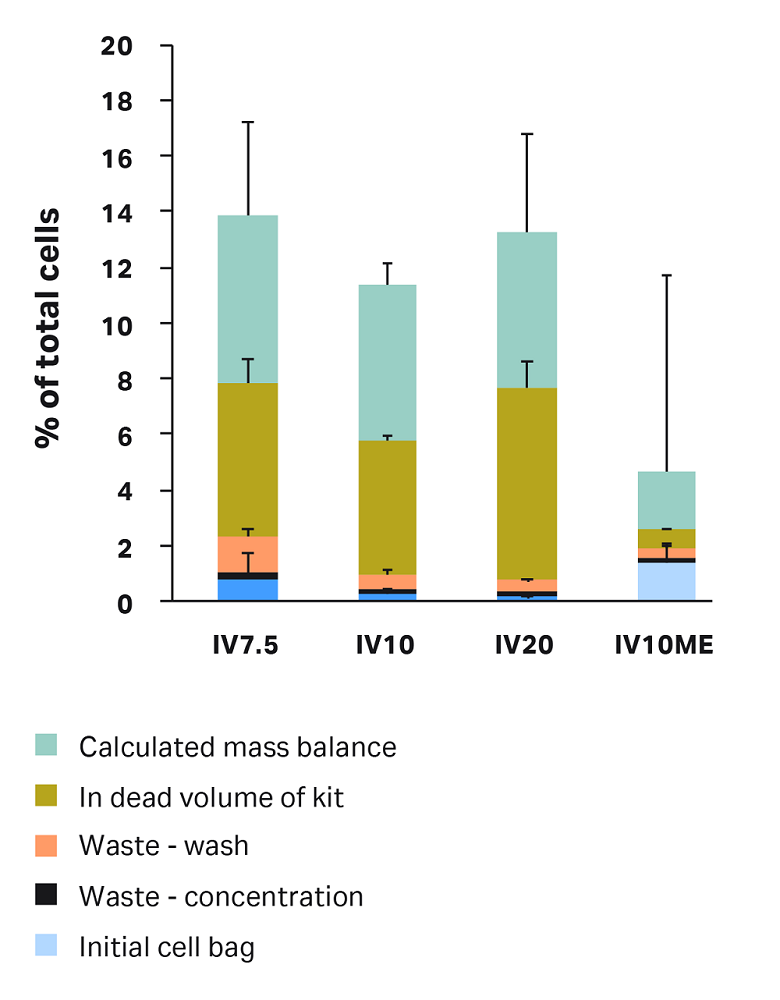
Fig 5. Overall cell loss in each condition by compartment. Mass balance is calculated as: 100% minus recovery minus the sum of all cell losses in each measured compartment. Each bar represents the average of technical triplicates ± SD.
Loss due to intermediate volume
The varying intermediate volumes for each test condition affect the percentage of cells in waste after wash. Here, we tested low (IV7.5), middle (IV10 and IV10ME), and high (IV20) intermediate volumes (Fig 6), corresponding to 12.5 mL, 10 mL, and 0 mL rinse volumes, respectively. Though loss in each condition was less than 1.4%, significantly more cells were lost with IV7.5 (1.3%) compared to IV10 (0.6%), IV20 (0.4%), and IV10ME (0.3%).
The set intermediate volume governs the volume of cell pellet left in the chamber during supernatant extraction and after the centrifuge steps. To minimize loss in the waste bag, we recommend setting the intermediate volume higher than the cell pellet volume. If the cell volume is not well defined, an additional parameter in the CultureWash C-Pro application — optical cell detection — will ensure minimal cell loss in the waste. Here, we did not enable optical cell detection to allow for testing of specific intermediate volumes.
The higher loss in waste after wash for IV7.5 indicates that an intermediate volume of 7.5 mL is near the limit for the cells, cell number, and buffer tested. Cells were visible in the line going toward the waste bag during the IV7.5 runs (not shown), validating this limit.
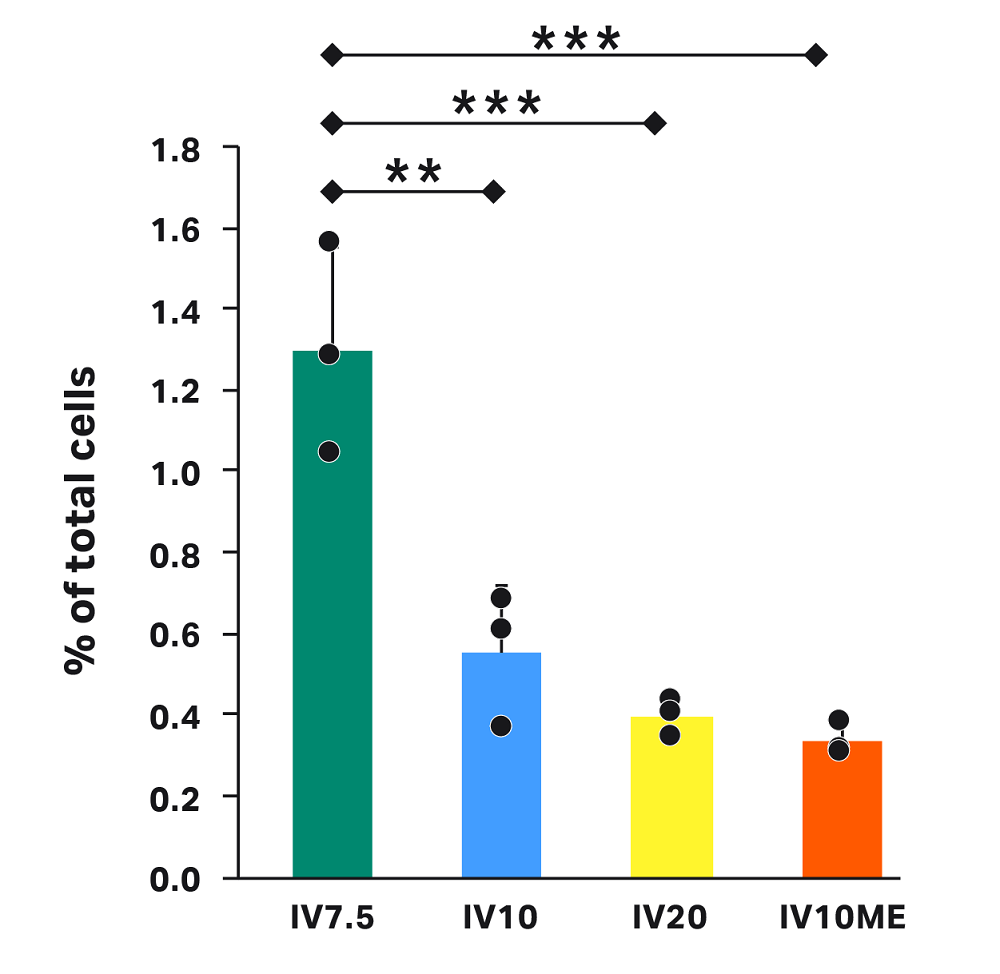
Fig 6. Cell loss in the waste bag after the wash step in each condition. Each vertical bar represents the average of technical triplicates ± SD, with each replicate shown in black. Horizontal bars represent statistical significance with ** for p < 0.01 and *** for p < 0.0001.
Loss in dead volume of kit
Without manual extraction, wash buffer containing some cells remains in the kit after processing (Fig 7). We measured the volume of the remaining solution (“dead volume”) for all runs in conditions without manual extraction (Fig 7). Because leftover volume was minimal when manual extraction was used, dead volume was only measured in one of the three manual extraction runs. As expected, there was no statistically significant difference in measured dead volume between the conditions where manual extraction was not used. The average dead volume, including IV7.5, IV10, and IV20 conditions, was 1.65 ± 0.27 mL.
To calculate percentage of total cells in the dead volume, we measured cell concentration for each dead volume sample (Fig 7). When manual extraction was used (IV10ME), the cells left in the dead volume was significantly lower than the cells left when manual extraction was not used (IV7.5, IV10, IV20), as expected. For conditions where manual extraction was not used, differences in the percentage of total cells were not statistically significant — though the loss in the dead volume was slightly higher in condition IV20 than in IV7.5 and IV10.
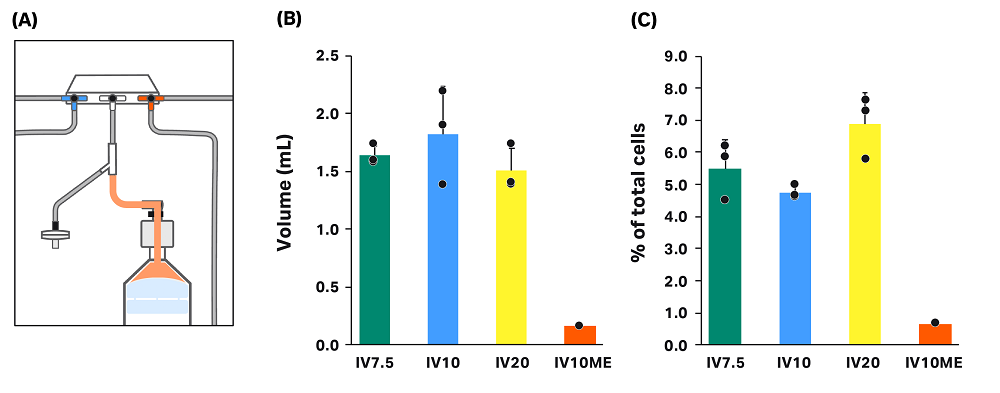
Fig 7. Cell loss in the dead volume of the kit. (A) Illustration of the CT-60.1 kit showing the dead volume location in orange. (B) Measured volume of the dead volume in each condition. (C) Cell loss in the dead volume as a percent of total cells in each condition. Each vertical bar represents the average of technical triplicates ± SD, with each replicate shown in black.
In the absence of manual extraction, the concentration of cells in the dead volume is partially dependent on the dilution of the cell pellet during the extraction to the final bag. Cells in the selected intermediate volume are first extracted to the final bag, leaving cell solution in the line between the final bag and the centrifuge chamber. Then, wash buffer is added to the chamber in the amount of the rinse volume. The rinse solution is then extracted to the final bag. After removal of the kit air filter from the Sepax C-Pro system, cell solution starting at the pressure sensor line can flow into the final bag, leaving solution in the dead volume only. If the final volume is sufficiently high, the cells left in the dead volume will be minimal. With a low final volume, however, there are more cells left in the dead volume. See Appendix A for theoretical calculations of cell loss in the dead volume.
These data indicate that the manual extraction option can help recover a significant number of cells, increasing the total recovery by ~4% to 7% with a 20 mL final volume. That said, the level of loss recovery with manual extraction can vary depending on the parameters used, the cell type, and the final cell density, among other variables, and therefore should be validated for each individual application.
Conclusion
In this study, we tested three conditions varying rinse volume without manual extraction and one condition using a manual extraction method on CultureWash C-Pro v432 with 1 ×1010 Jurkat cells and a final volume of 20 mL. Overall, > 86% recovery was achieved, with 95% average recovery when manual extraction was used and high viability across all conditions.
The largest measurable cell loss was in the dead volume of the kit. Using manual extraction significantly reduces the dead volume loss, increasing final cell recovery. We therefore recommend using a manual extraction method for final volumes of 20 mL or less. Up to 6% of total cells were still unaccounted for after evaluating cells left in all bags and in the dead volume.
The rinse volumes tested did not have a significant impact on cell recovery, though the loss was slightly higher with a 0 mL rinse (IV20) compared to 10 mL (IV10) and 12.5 mL (IV7.5) rinse volumes.
We tested three intermediate volumes. Though loss due to intermediate volume was low in each condition, loss in the waste after wash was significantly higher with a 7.5 mL rinse, indicating that 7.5 mL is near the low limit for 1 × 1010 Jurkat cells under the tested conditions.
Parameter guidelines for cell processing on CultureWash C-Pro
Using the data obtained with the model cell line in this study and under the limited conditions tested here, we define general guidelines to maximize recovery below.
Manual extraction: Cells left in the dead volume depend on the cell concentration during the final extraction to the waste bag. See Appendix A for theoretical calculations relating the rinse and final volumes to the cells left in the dead volume. We recommend manual extraction for final volumes of 20 mL or less, regardless of rinse volume.
Rinse volume: Here, the different rinse volumes tested did not significantly change the overall cell recovery. However, slightly different losses in dead volume provided valuable insight to rinse volume guidelines. Dead volume loss was slightly higher with a 0 mL rinse volume (IV20) compared to 10 mL (IV10) and 12.5 mL (IV7.5) volumes. This data and the theoretical calculations presented in Appendix A led us to recommend a rinse volume of at least 5 mL.
Intermediate volume: These data suggest that a 7.5 mL intermediate volume is a lower limit for 1 × 1010 Jurkat cells in the buffer used and under the centrifuge parameters chosen for this study. The intermediate volume is dependent on individual conditions. Further investigation is needed to better understand the impact of individual parameters (including cell type, cell size, wash buffer, g force, and centrifuge time) on the lower limit of the intermediate volume. We recommend enabling the Optical Cell Detection parameter in the CultureWash C-Pro application to minimize loss due to incorrect intermediate volume setting.
Ordering information
| Product | Product code |
|---|---|
| Xuri Cell Expansion System W25 | 29064568 |
| Xuri Cell Expansion System Cellbag Perfusion, pH and DO, 10L | 29105499 |
| RPMI 1640 medium without L-glutamine | SH30096.02 |
| Defined Fetal Bovine Serum, US Origin | SH30070.03 |
| Penicillin-Streptomycin solution | SV30010 |
| SG-200 | SH3059001 |
| Sepax C-Pro Cell Processing Instrument | 29264741 |
| CultureWash C-Pro Protocol Software | 29264736 |
| CT-60.1 Sepax C-Pro Cell Processing Kit | 29264739 |
| CT-90.1 Sepax C-Pro Cell Processing Kit | 29264740 |
Appendix A: Theoretical calculations to assess loss in dead volume for different rinse and final volumes
*Note: This theoretical model is based on limited data. It is meant solely for the purpose of supporting customers in narrowing down their parameter testing and should be practically vetted for each sponsor’s specific applications.
In the absence of manual extraction, the percent of total cells left over in the dead volume is dependent on the concentration and dilution of the cells during extraction to the final bag. The extraction happens in two steps: first, the cells in the set intermediate volume are moved to the final bag; then, the chamber is rinsed in the remaining volume (the “rinse volume”) to achieve the set final volume. With known rinse and intermediate volumes, you can calculate theoretical loss using this equation:
Equation 1:
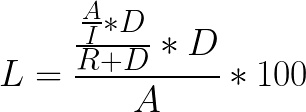
Where
L = % loss
A = Total cells
I = Intermediate volume
D = Dead volume
R = Rinse volume
In this study, dead volume was measured as 1.65 ± 0.27 mL. The percent loss is independent of the number of total cells, so A can be removed from the equation. The intermediate volume variable can be replaced using the equation I = F - R. The final equation when rinse volume is greater than 0 mL is:
Equation 2:
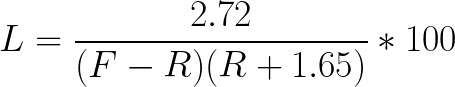
When rinse volume is 0, the theoretical loss is represented by the equation:
Equation 3:
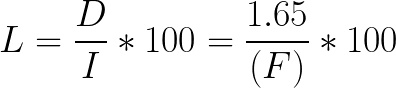
Using Equations 2 and 3, we graphed the theoretical loss for varying rinse and final volumes (Fig A1). In this figure, you can see that loss is higher when rinse volume is less than 5 mL and when the final volume is 20 mL or less. Because of this finding, we recommend using manual extraction when the final volume is 20 mL or less. When the volume is greater than 20 mL, use a rinse volume of at least 5 mL.
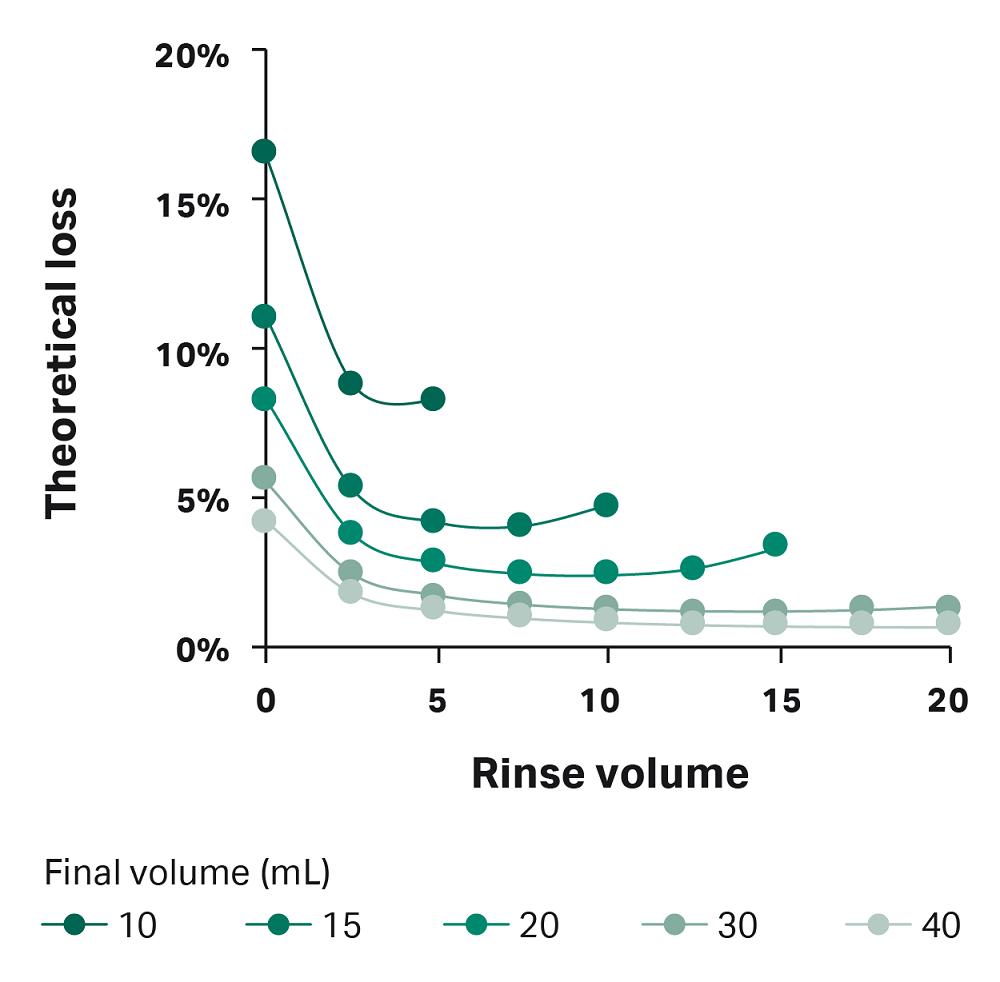
Fig 1A. Graphical representation of Equations 2 and 3, showing theoretical loss in dead volume. Lines represent different final volumes. Note: This estimated loss is theoretical. Actual loss measured in the dead volume in this study was similar to the estimation for IV20, but higher than the loss estimated here for conditions IV7.5 and IV10.