Introduction
Nature is incredibly smart, creative, and efficient in so many ways, and the human immune system is a testament to that (1). As we’ve begun to slowly understand and unlock the complexities of immune mechanisms through technology, we’ve found better ways to fight disease. Immunotherapy of cancer is a prime example – it’s based on natural immune defenses that can be optimized to target specific cancers. Here, we focus on an important player in such targeted, highly personalized cancer therapies called tumor-infiltrating lymphocytes (TILs).
Two modes of immunotherapy for cancer have garnered much attention in the past decade: immune checkpoint inhibitors and adoptive cell transfer (ACT). TILs belong to the latter, along with chimeric antigen receptor (CAR) T cells, and are used to treat late-stage patients, including those patients who don’t respond to checkpoint inhibitor drugs. CAR T cell therapy is perhaps the better-known ACT modality, but TILs have proved more promising in treating certain solid tumors. This article will look at the tapped and untapped potential of TILs in cancer care, as well as the limitations of their use and some challenges in therapy development.
What are tumor-infiltrating lymphocytes?
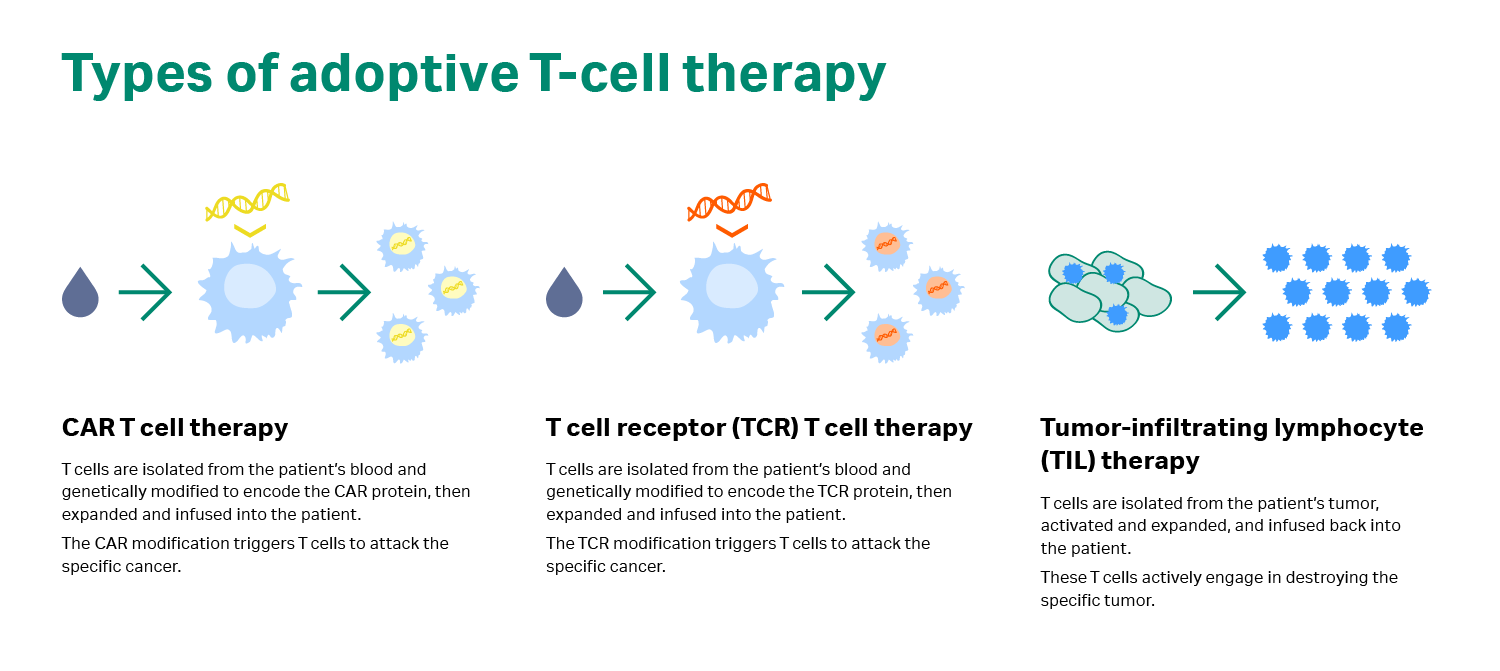
In adoptive cell transfer or ACT, immune cells are removed from the body, expanded in the lab, and then returned to the patient. The ACT approach uses one of two strategies to destroy tumors: naturally occurring TILs from solid tumor masses or genetically modified T cells that recognize specific tumor cells (i.e., CAR T cells and TCR modified T cells; see Fig 1) (2).
TILs are a naturally occurring, heterogeneous population of lymphocytes (white blood cells) that migrate into a tumor, and they may be the most relevant immune cells in the body for fighting that cancer. These cells mostly comprise T cells that actively engage in tumor destruction. So, how can we harness and target the cytotoxic ability of TILs to fight cancer?
“TILs enter into the tumor to eradicate it, but for some reason their activity has been stopped; maybe because there’s too few of them, maybe due to some immunosuppressive mechanism within the tumor microenvironment – we know this has a huge impact on the infiltrating lymphocytes,” responds Dr. Özcan Met, Associate Professor and Head of Cell Manufacturing at the National Center for Cancer Immune Therapy, University Hospital Herlev in Denmark. “So, the idea with TIL therapy is to extract these cells from the tumor, activate them, increase them in number, and reintroduce them to the patient to generate a robust immune-mediated anti-tumor response.”
Fig 1. Types of adoptive cell transfer.
Why are TILs important in immunotherapy for cancer?
The clinical benefit of TILs was observed as early as 1972, many years before they were developed into the kind of therapy used today (3). It is now believed that TILs may very well be present in many solid cancers, and their presence is often associated with a favorable prognosis, according to Dr. Met. But how do TILs compare to CAR T cells? The key fact to understand here is that both these therapy forms are effective and have their own merits, but for different indications. “It’s not one or the other,” says Dr. Met.
While TIL therapy takes advantage of the natural, tumor-specific T cells that exist in the patient, CAR T cells are genetically modified to obtain specificity to a certain antigen. Another difference is the source of the cells: TILs are extracted from solid tumors, while T cells later modified with a CAR are extracted from peripheral blood. In terms of clinical response, TILs have shown clear efficacy in one solid cancer type – melanoma (4), and there’s more research now into other solid cancer types, such as breast, cervical, and ovarian cancer (5‒7). CAR T cells have been very effective in hematological malignancies that present CD19, an antigen exclusively expressed on the surface of B cells (another type of immune cell), both normal and cancerous. Particularly in acute lymphoblastic leukemia (ALL), CAR T therapy has demonstrated complete response rates of 70% to 90%, as reported by multiple institutions (2).
Because CAR T therapy can affect normal B cells, there is a major side effect termed ‘on-target off-tumor toxicity’. But this is a manageable problem, according to Dr. Met. The patient can be given immunoglobins before and after the therapy to manage B cell depletion (B cell aplasia). However, this type of side effect could be fatal if the antigen was expressed on cells in essential organs, such as the liver or heart. Hence, there is a considerable barrier for the use of CAR T therapy to treat solid cancers.
“When it comes to TILs, it's very important to stress that the major focus has so far been on melanoma. We are starting to slowly investigate the use of TILs in other cancer forms, but most of the proof-of-concept has been done in melanoma, particularly by Dr. Steven Rosenberg’s group at the National Cancer Institute (NCI),” notes Dr. Met. “On the other hand, the proof-of-concept for CAR T cells has been in cancers like B cell lymphomas and lymphocytic leukemia.” Essentially, the applications have fallen into different buckets so far.
An interesting feature of TILs that also points to their significance in cancer therapy is antigen recognition. TILs contain T cell receptors (TCR) that recognize various intracellular proteins and shared antigens expressed in many different organs as well as cancer tissue. Thus, antigen selection is difficult with TILs, compounded by the fact that the tumor microenvironment is much more immunosuppressive in solid cancers than in cancers formed in blood or blood-based organs. But the silver lining here is that TILs can also recognize neoantigens – antigens that are very specific for each patient, because these antigens result from unique mutations acquired during cancer development. As such, it’s possible to optimize TILs to target them to a certain neoantigen. According to Dr. Met, this type of optimization towards neoantigen recognition is at the leading edge of TIL therapy research currently.
How TIL therapies are developed and the associated challenges
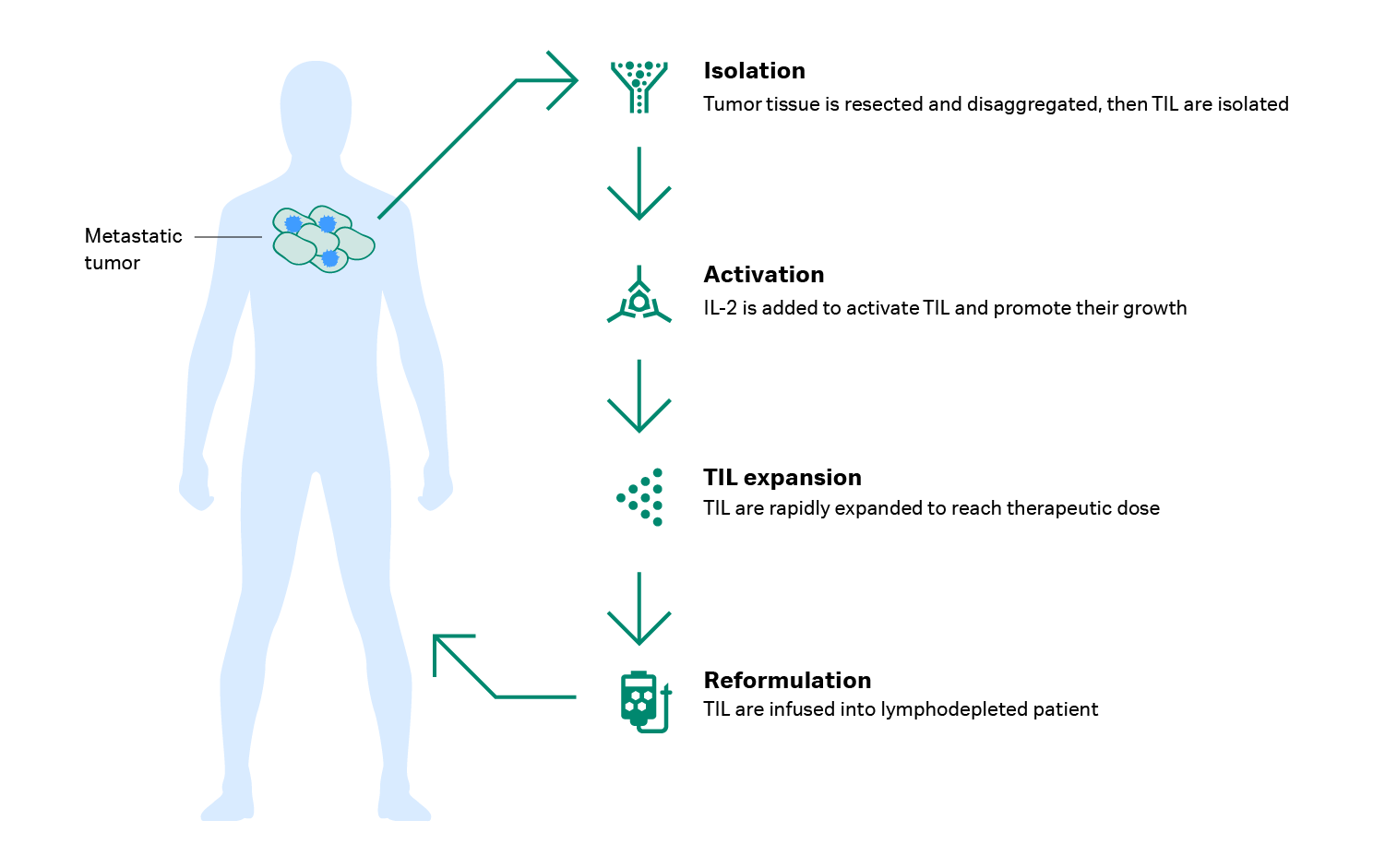
Developing a TIL therapy starts with collecting a tumor tissue sample and extracting the lymphocytes from it. Because these cells are present in very low numbers, they must be carefully handled and expanded. Dr. Met explains that you can obtain T cells from a resected tumor in two ways: “You can either take the tumor and do a tissue digest to get a single-cell suspension, or you can cut the tumor into fragments that you can grow in plates.”
During TIL culture, high doses of interleukin-2 (IL-2), a growth factor, is given to activate the TILs and promote their growth. Then the TILs are expanded using a rapid expansion protocol pioneered by Dr. Steven Rosenberg and his colleagues at the Surgery Branch of the NCI. “It’s a 2-week expansion,” says Dr. Met. “The first week is a static phase, and the second week is a dynamic phase. We initiate the cell expansion on day 0 using anti-CD3 antibody, allogeneic feeder cells, and a high dose of IL-2. In our case, we typically go from 20 million TILs to an average of 100 billion TILs. This is a 5000-fold expansion within two weeks.”
Before the TILs are returned to the patient, the patient is given a high dose of chemotherapy to remove some nonspecific immune cells and ‘make room’ for the infusion of TILs – this is called lymphodepletion and it’s followed by another dose of IL-2 (8). “The lymphodepletion is crucial for getting a significant effect,” Dr. Met says. “Without preconditioning the patient with lymphodepletion, some groups have failed to show high clinical response.” Figure 2 provides an overview of TIL therapy development.
Fig 2. How TIL therapy is developed.
The intensive nature of this treatment and the fact that it’s highly specialized are some of the main limitations of TIL therapy. Trained personnel are required to handle the TILs, ensure cell growth, and successfully turn around a product. Also, production requires a good manufacturing practices (GMP) grade facility, and the clinic must be tailored specifically for patients receiving this therapy. Dr. Met explains that it’s difficult to establish these facilities in a normal hospital setting, “there’s only one dedicated center in Denmark for this.”
Then there are the time and cost factors – developing the TIL therapy takes four to eight weeks. But some late-stage patients don’t have that long, and most of the ‘cost’ of TIL therapy comes from the time it takes to produce the cells. Dr. Met believes there are opportunities to bring down the time, and consequently the cost, by exploiting technology to optimize things like sample processing.
However, compared to CAR T therapies, producing TIL therapies may actually be cheaper, at least in an academic setting. “Currently, making CAR T cells is more expensive because of the viral vector that you put into the cells,” Dr. Met reflects. “With TILs, there is no genetically modified component and no use of viral vectors. What you need are some antibodies and allogeneic feeder cells, which are not as expensive, comparatively.”
Advancing TIL therapy: what the future looks like
As mentioned earlier, TIL therapy has shown significant, durable success in treating melanoma, in multiple studies (2). Dr. Met’s team in Denmark has explored TIL therapy for melanoma in combination with other treatment options like checkpoint inhibitors. ”We have seen that patients previously given checkpoint inhibitors are still responding to TIL treatment, and responding better than just the checkpoint inhibitor monotherapies,” he notes. Now, the focus is on expanding this success to other cancer types. Dr. Met and colleagues are testing TILs on ovarian cancer, using the same expansion method but pretreating and priming the patients with checkpoint inhibitors. They are also researching ways to make TILs more potent, cytotoxic, and specific, particularly for cancers that can see benefit from neoantigen-specific TILs.
“We are working on having two targeting modalities with TILs: on the one hand you have those TILs that recognize shared antigens broadly, and then on the other hand you have TILs that specifically recognize certain mutations unique to a patient – and that’s our research focus going forward,” explains Dr. Met. “We may have a hundred billion cells after TIL expansion, but the number of cells that actually do recognize and attack the tumor are much less. So, the trend is towards developing more specific TILs.”
Right now, TILs are being used as a salvage treatment for late-stage cancers, after first- and second-line treatment with checkpoint inhibitors (2). Dr. Met points out that, “if we can move TIL therapy to an earlier stage, we might derive even more benefit from it.” At the rate medical technology is progressing, it wouldn’t be a stretch to say we’ll see such developments in the near future.
References
- Petrányi GG. The complexity of immune and alloimmune response. Transpl Immunol. 2002 ;10(2-3):91‐100.
- Met Ö., Jensen KM, Chamberlain CA et al. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019;41:49‐58.
- Rosenberg SA, Fox E, Churchill WH. Spontaneous regression of hepatic metastases from gastric carcinoma. Cancer. 1972;29:472‐474.
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676‐1680.
- Zacharakis N, Chinnasamy H, Black M. et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nature Medicine. 2018;24(6):724‐730.
- Rosa K. Encouraging responses seen with TIL therapy in advanced cervical cancer. Targeted Oncology. 2019. Accessed June 10, 2020.
- Deniger DC, Pasetto A, Robbins PF, et al. T-cell Responses to TP53 "Hotspot" Mutations and Unique Neoantigens Expressed by Human Ovarian Cancers. Clin Cancer Res. 2018;24(22):5562‐5573.
- Rohaan MW, van den Berg JH, Kvistborg P. et al. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018:6;102.