Custom lateral flow assay development
Lateral flow immunoassays are high performing, easy-to-use, and cost effective. Invitro diagnostics manufacturers widely use these assays in rapid point of care testing and often need customized components as part of their products.
Thoughtful lateral flow assay design and development is key to high performance and minimized costs, achieved in part by selecting the best components for optimal assay results.
What are lateral flow assays?
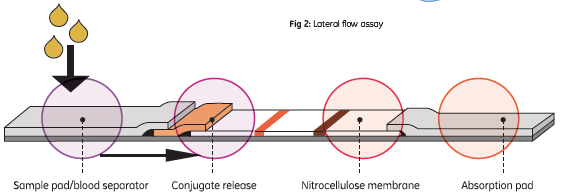
Lateral flow immunoassay systems are generally single-step assays, requiring only the addition of a sample. They consist of a sample pad or blood separator, conjugate release, nitrocellulose membrane, and an absorption pad (Fig 1).
Fig 1. Schematic of typical lateral flow assay.
The sample flows from the sample pad/blood separator through to the conjugate release and contacts dried reagents, usually a tagged secondary antibody. The antibody and analyte then migrate to a capture zone of nitrocellulose membrane-immobilized antibody. Any unreacted tagged antibody flows past the capture zone to the absorption pad.
Properties of the components—particularly wicking rates—affect the accuracy and reliability of lateral flow assays, highlighting the need to consider each component when designing a custom assay.
Selecting the sample pad/blood separator
Appropriate choice of sample pad or blood separator ensures an assay begins without complications. Sample pads are usually either cotton linter or bound glass fiber.
Cotton linters are well suited for small sample volumes (up to approximately 200 µL) and has a slower wicking rate than bound glass fiber. Glass fiber sample pads do not cause red cell hemolysis and so are suitable as blood separators.
Other considerations when choosing a sample pad or blood separator:
- Consistent absorbency and wicking rates: Ensures test-to-test reproducibility.
- Low protein binding: Minimizes loss of analyte, maintaining test sensitivity.
- Manufactured in controlled conditions: ISO 9001 certified environments protect components from contamination during manufacture.
Conjugate release pad considerations
Conjugate release pads are critical to the performance of lateral-flow immunoassays. In the pads the conjugates should dry and be stored without damage or aggregation. Then they should be and released rapidly when the sample comes into contact.
Selecting the best-suited conjugate release pads saves on both costs and time. For example, inherently hydrophilic pads will not require treatment before conjugate application, reducing reagent costs. Selecting material that has an open structure allows fast penetration by both conjugate and sample, further saving time.
Properties to look for when selecting a conjugate release pad:
- High levels of conjugate release: Leads to less waste and reduced reagent costs.
- Natural and rapid pad rewetting: Provides improved consistency after prolonged storage.
Nitrocellulose membrane selection
Nitrocellulose membranes are a key part of lateral flow assays, because they notably impact test sensitivity. These membranes are available in a range of wicking rates and formulations.
A sharp and intense capture line relies on the nitrocellulose membrane’s ability to bind sufficient protein while minimizing background levels for easy interpretation of results.
Considerations when selecting a nitrocellulose membrane:
- Sample viscosity: High viscosity samples require a lateral flow membrane with a high wicking rate.
- Assay optimization: Unbacked membranes enable use of either belt or air side of the membrane, simplifying optimization.
- Mechanical strength: Beneficial for reel-to-reel machines, backed membranes have higher strength.
Absorption pad properties
Absorption pads at the end of the tests control sample flow along the strip. Appropriate wicking characteristics for the type of sample and assay produce increased test-to-test consistencies. Ensuring the absorbent has sufficient capacity is also a consideration when designing an immunoassay.
What to look for:
- Consistent absorbency: Ensures test-to-test reproducibility.
- Manufacturing in controlled environment: ISO 9001 certified environments minimize the risk of false results due to contamination.
- Minimal leakage along strip: Protects against contamination of test results.
- Rapid rewetting: Maximizes test consistency.
What is best for my assay?
Cytiva is an established technology component provider for point-of-care lateral flow immunoassays. We produce a wide range of cellulose and glass fiber substrates and nitrocellulose membranes to an assured quality for accurate and reproducible results.
Our custom services team can identify your needs and supply cost-saving customized solutions via a personalized service. Our experts will help you identify and optimize components, ascertain the best-suited technologies, and offer invaluable assistance. To discuss any challenges you are facing, please contact Scientific Support. or try our Whatman Filter Selector App to find out if you are using the most appropriate filtration solution for your samples.