ÄKTA go protein purification system has been developed for automated chromatography from the heritage of our fast protein liquid chromatography (FPLC) technology. With flow rates of up to 25 mL/min and pressure of up to 5 MPa, you are all set for affinity, size exclusion, or ion exchange chromatography. Here we describe an antibody purification as well as purification of two tagged proteins using a histidine tag (his tag) and a Strep-tag® II.
1. Antibody purification
In this example, a monoclonal antibody (mAb) was purified from a Chinese Hamster Ovary (CHO) cell culture extract using two chromatography steps. In the first step, affinity purification using a HiTrap Protein G HP antibody purification column was used. The eluate was passed on to a second chromatography step using a HiPrep 16/60 Sephacryl S-300 HR size exclusion chromatography column to further improve purity.
| Sample: | mAb expressed in CHO cells |
| Sample prep: | Clarified by filtration |
| Affinity chromatography: | HiTrap Protein G HP column, 1 mL (Buffer A: 20 mM sodium phosphate buffer, pH 7.0, Buffer B: 50 mM glycine-HCl buffer, pH 2.5) |
| Size exclusion chromatography: | HiPrep 16/60 Sephacryl S-300 HR (Buffer A: PBS) |
Predefined methods simplify and speed up the purification
ÄKTA go is fully supported by UNICORN software and gives real-time control of the chromatography system. In this application the methods in Figure 1 were generated using such predefined methods.
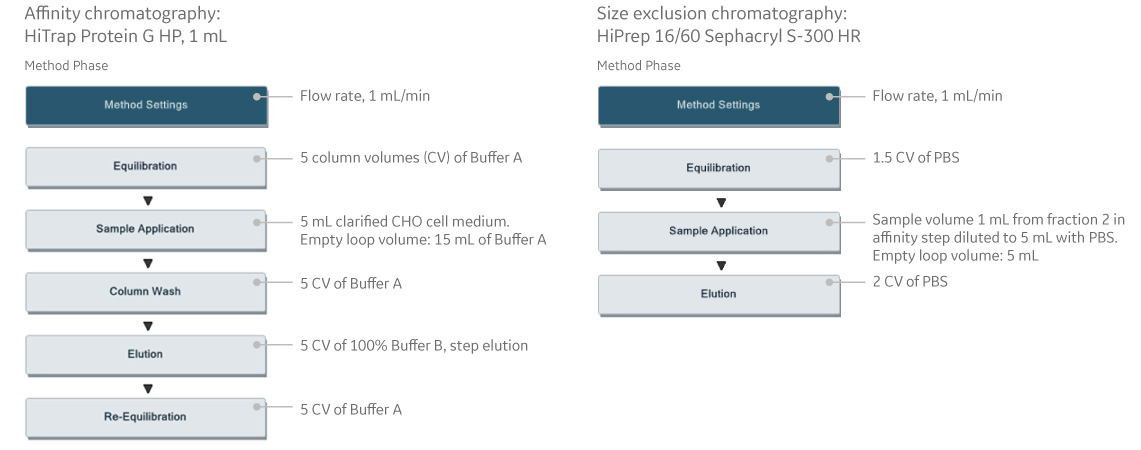
Fig 1. Predefined methods for affinity chromatography and size exclusion chromatography steps in UNICORN software.
Two-step antibody purification isolates mAb monomers from aggregates and fragments
In the chromatograms shown in Figures 2 and 3, results from the two-step protocol are shown.
Affinity chromatography step: HiTrap Protein G HP 1 mL
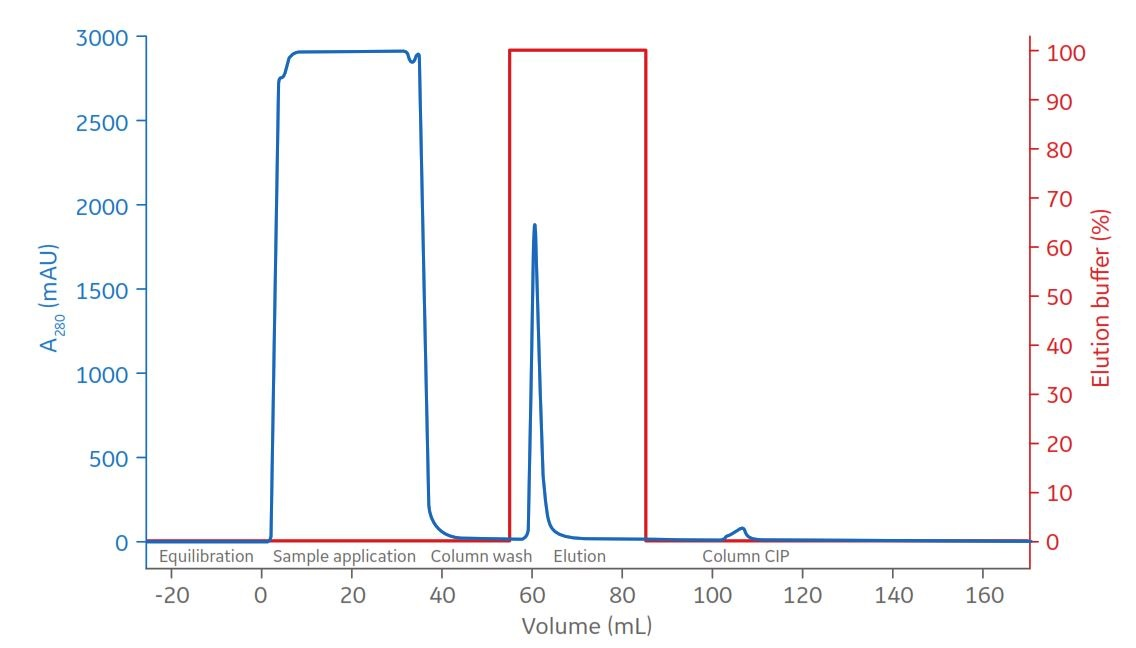
Fig 2. A 5 mL sample of clarified CHO medium applied to HiTrap Protein G column. Protein G bound material was eluted by step elution with buffer B containing 50 mM glycine-HCl at pH 2.5.
Size exclusion chromatography step: HiPrep 16/60 Sephacryl S-300 HR
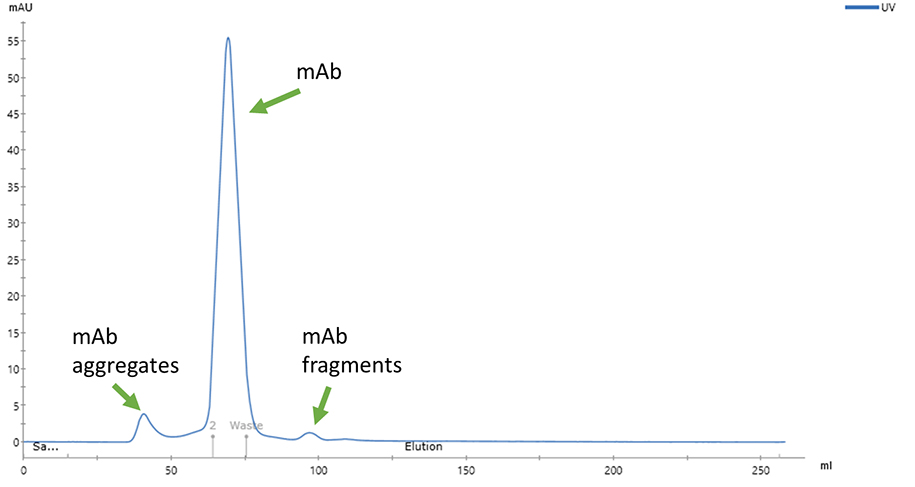
Fig 3. Fraction 2 from the affinity (Protein G) chromatographic step was applied on the SEC column. SEC isolates the antibody monomers from aggregates and fragments.
Tips for antibody purification
- Identify the optimal affinity capture resin with respect to species and subclass selectivity.
- If the sample is not pure enough after the SEC, optimize the affinity step or add an extra intermediate purification step, for example, ion exchange chromatography.
- The collected fractions from the affinity step should be neutralized immediately to preserve activity.
- Select the appropriate chromatography resin, format, and instrument that meets your needs.
2. His tag protein purification
His-tagged green fluorescent protein (GFP-His) was purified from an E. coli cell extract using two chromatography steps. The columns for each step were connected to the ÄKTA go system at the same time using the column valve (Fig 4).
In the first step, affinity purification using a HisTrap HP column, a HiTrap column prefilled with Ni Sepharose High Performance resin, was used. The eluate was passed on to a second chromatography step using a HiLoad 16/600 Superdex 75 pg column to further improve purity. Other SEC columns such as HiPrep Sephacryl S-200 HR can be used depending on, for example, the purity requirements
Fig 4. HisTrap and HiLoad columns were mounted at the same time on ÄKTA go using the optional column valve.
Predefined methods simplify the purification
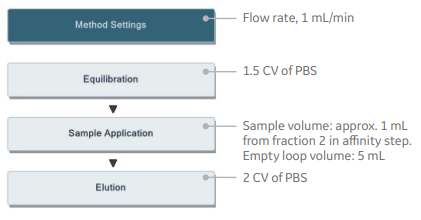
In this application, the methods below were generated using predefined methods (Fig 5).
Affinity chromatography:
HisTrap HP, 1 mL
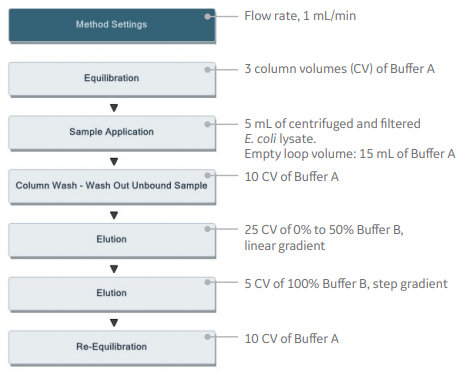
Size exclusion chromatography:
HiLoad 16/600 Superdex 75 pg
Fig 5. Predefined methods for affinity chromatography and size exclusion chromatography steps in UNICORN software.
Efficient and reliable his tag protein purification
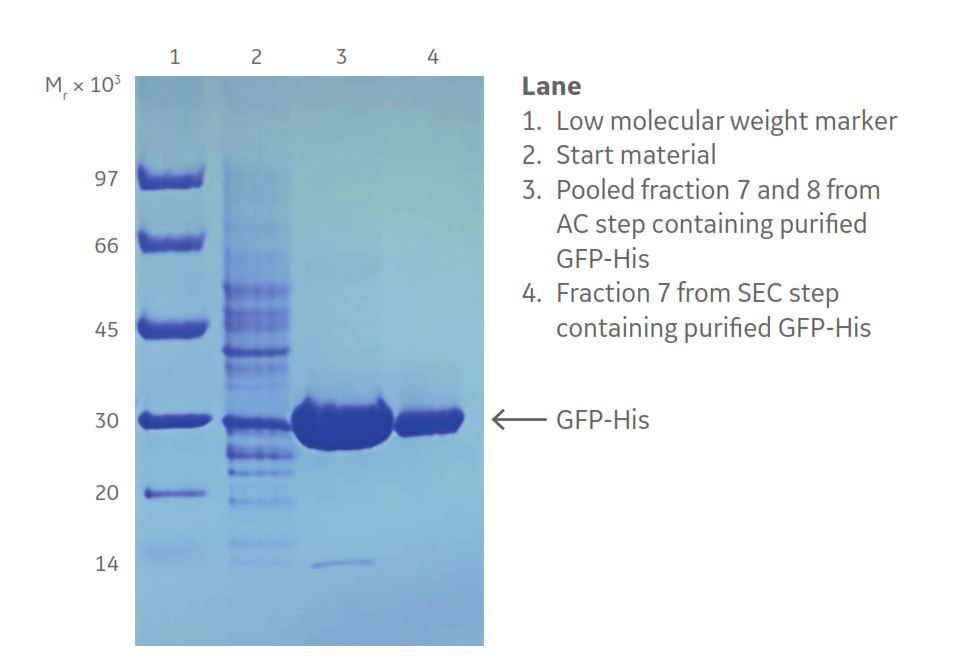
His-tagged protein purification simplified protein purification and enabled the use of standard protocols. In Figures 6 and 7, results from the two-step protocol are shown. The results from SDS-PAGE verified that GFP-His was effectively purified after the affinity chromatography step even though some smaller impurities were removed after the final SEC step (Fig 8).
| Sample: | GFP-His expressed in E. coli cells |
| Sample prep: | Frozen paste was resuspended, sonicated, and centrifuged; 10 mL of the supernatant was filtered through a 0.45 µm filter |
| Affinity chromatography: | HisTrap HP, 1 mL (Buffer A: 20 mM sodium phosphate, 500 mM NaCl, pH 7.4; Buffer B: 20 mM sodium phosphate, 500 mM NaCl, 500 mM imidazole, pH 7.4) |
| Size exclusion chromatography: | HiLoad 16/600 Superdex 75 pg (Buffer A: PBS) |
| Analysis | SDS-PAGE |
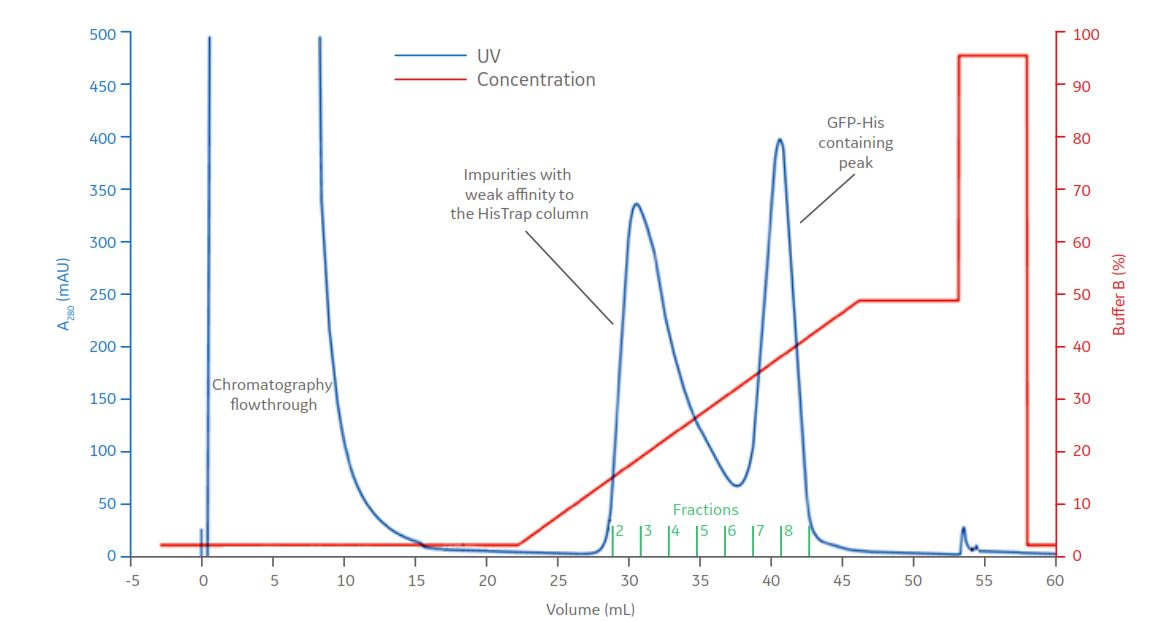
Affinity chromatography step: HisTrap HP 1 mL
Fig 6. The 5 mL sample of E. coli lysate containing GFP-His was applied on a HisTrap HP column. Bound material was eluted by a linear gradient of up to 50% of Buffer B containing 20 mM sodium phosphate, 500 mM NaCl, and 500 mM imidazole. Impurities with weaker affinity to the resin eluted at lower imidazole concentration compared to GFP-His. Fraction 7 and 8, containing GFP-His, were pooled and applied on a HiLoad 16/600 Superdex 75 pg SEC column.
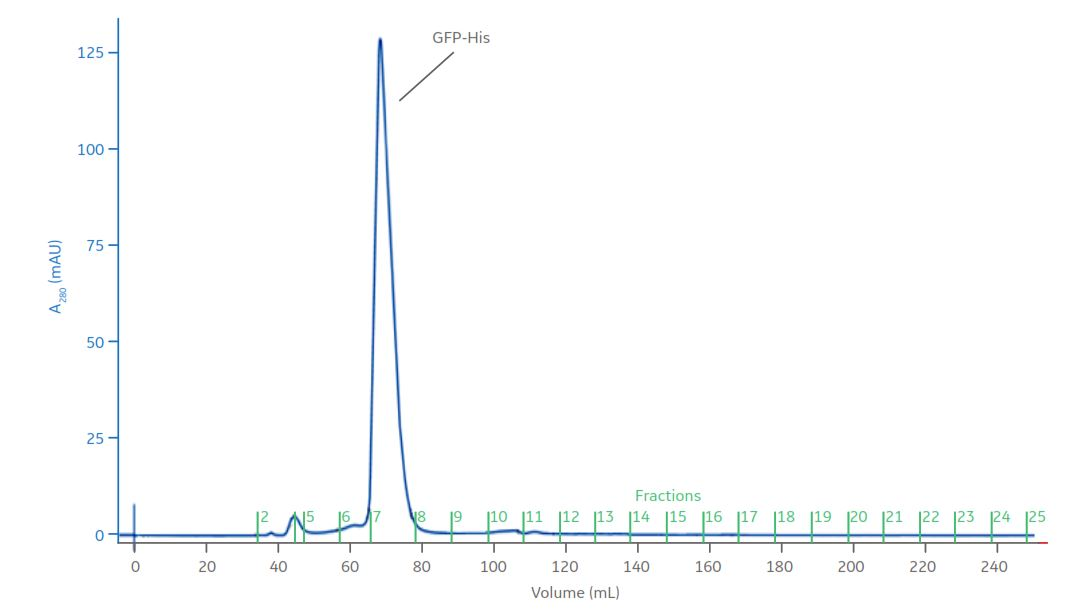
Size exclusion chromatography step: HiLoad 16/600 Superdex 75 pg
Fig 7. Fractions 7 and 8 from the affinity chromatography step were pooled to make a total volume of 3.8 mL. The pooled fraction was applied on the HiLoad 16/600 Sephadex 75 pg SEC column.
Fig 8. Coomassie™ blue stained SDS-PAGE gel showing GFP-His purity after the two chromatographic steps. Lane 4 shows the purified GFP-His.
3. Strep-Tactin® XT Sepharose for affinity purification of recombinant proteins
Strep-tag II is a small (8 amino acid) tag that can be placed at the C- or N-terminus of the target protein to simplify protein capture and purification. The small molecular size of the tag means that it does not affect target protein integrity, which minimizes the need for tag removal after purification. Strep-tag II and the tandem version, Twin-Strep-tag® can be used in physiological conditions, resulting in a tag system that is suitable for a wide range of proteins.
Strep-Tactin XT Sepharose is a further development of StrepTactin Sepharose High Performance (HP) resin offering higher affinity and enabling purification under denaturing conditions. Strep-Tactin XT is available in 1 and 5 mL prepacked columns as well as bulk resins. The Strep-Tactin XT ligand gives higher protein yield and minimizes loss of valuable target protein. In addition, the high binding specificity allows high target protein purity in only one purification step.
GFP-Twin-Strep-tag was purified from an E. coli cell extract using a StrepTrap XT column. Strep-Tactin XT is a specially engineered streptavidin ligand. Twin-Strep is a sequential arrangement of two Strep-tag II sequences with a binding affinity to Strep-Tactin XT in the low picomolar (pM) range with high specificity.
Single-step purification of Strep-tag II protein
The use of the Strep-tag II recombinant protein simplified purification and allowed purification using a single-step purification (Fig 9). High yield (29.2 mg) and high purity were achieved using the 5 mL StrepTrap XT column. Predefined methods simplified method creation and enabled quick start up.
| Column | StrepTrap XT 5 mL |
| Sample: | Glyceraldehyde 3-phosphate dehydrogenase (GAPDH Strep-tag II, tetramer, Mr 37 400/subunit), 1 mg/mL in E. coli lysate |
| Sample prep: | Frozen paste was resuspended, sonicated and centrifuged. 10 mL of the supernatant was filtered through a 0.45 µm filter |
| Sample volume: | 30 mL |
| Binding buffer: | 100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, pH 8.0 |
| Elution buffer: | 100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 50 mM biotin, pH 8.0 |
| Regeneration: | 3 column volumes (CV) distilled water, 3 CV 50 mM NaOH, 3 CV distilled water |
| Flow rate: | 5 mL/min |
| System: | ÄKTA go |
| Analysis | SDS-PAGE |
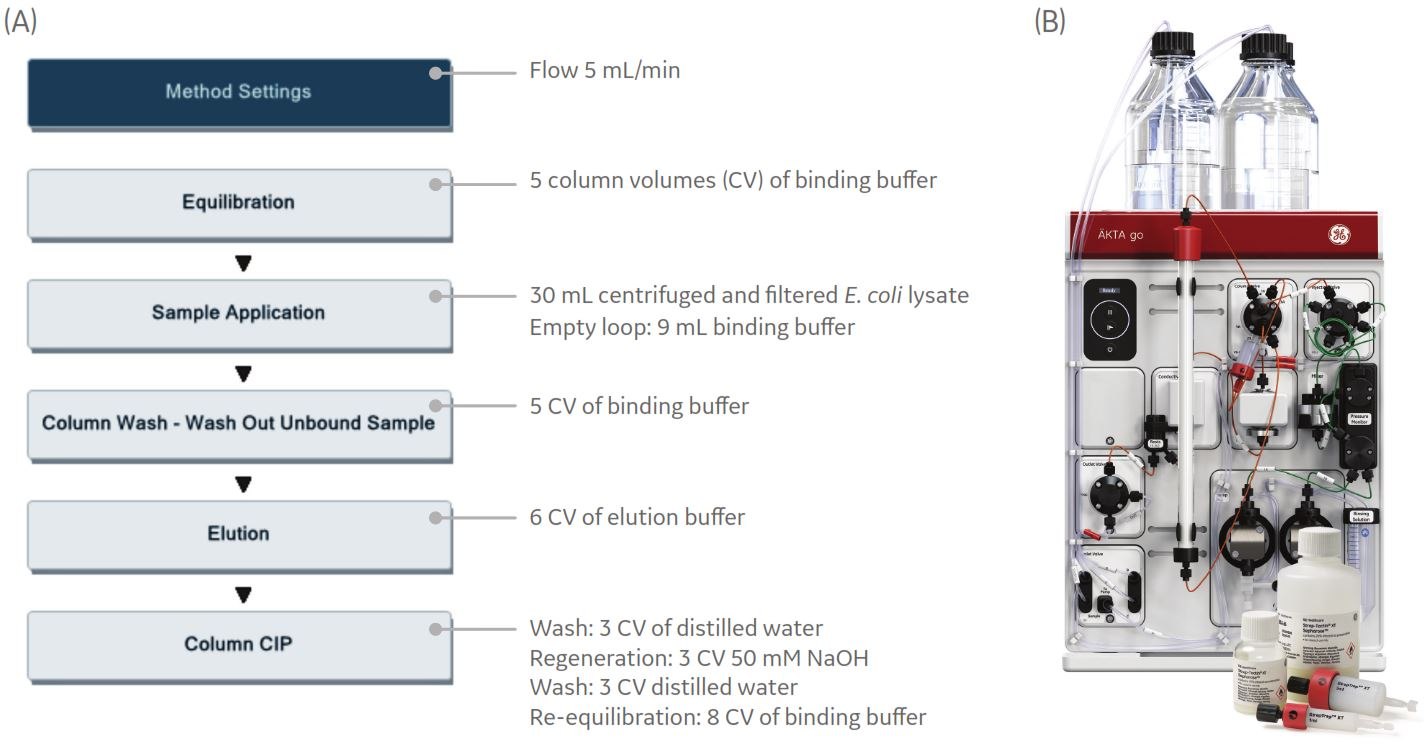
Fig 9. (A) Predefined method for affinity chromatography in the UNICORN software. Column regeneration with three different solutions was performed in a CIP phase using an optional Inlet A valve. (B) ÄKTA go protein purification system, StrepTrap XT columns, and Strep-Tactin XT Sepharose affinity chromatography resin.
The use of the Strep-tag II recombinant protein simplified purification and allowed purification using a single-step purification (Fig 10). High yield (29.2 mg) and high purity were achieved using the 5 mL StrepTrap XT column. The results from the SDS-PAGE verified that GFP-Twin-Strep-Tag was effectively purified by the StrepTrap XT affinity technique. Due to its high binding specificity, only one purification step is required to reach high protein target purity.
Affinity chromatography step: StrepTrap XT 1 mL
Fig 10. Chromatogram showing the purification of 30 mg of GAPDH Strep-tag II on StrepTrap XT 5 mL column.Tips for tagged protein purification.
Tips for tagged protein purification:
- Select a tag based on your purification priorities, and size of the tag.
- Define the required level of purity and identify options to achieve this level of purity.
- If the sample is not pure enough after the affinity step, add an extra purification step, e.g., size exclusion chromatography.
- Determine whether to remove the tag and how.
- Select the appropriate chromatography resin, format, and instrument that meets your needs.
Resources for more information: antibody purification
- Selection guide: Affinity chromatography columns and media (pdf)
- Selection guide: Columns and resins for antibody purification and immunoprecipitation
- Affinity chromatography Vol.1: Antibodies (pdf)
Resources for more information: tagged purification
- Purifying tagged proteins
- Affinity chromatography handbook Vol.2: Tagged proteins
- How to guides online: Protein purification methods