We optimized influenza A virus production from anchorage‑dependent Vero cells using Cytodex™ 3 and Cytodex™ 3 Gamma microcarriers in the ReadyToProcess WAVE™ 25 bioreactor. By using the system’s smoother rocking motion (100%), we minimized shear stress, enabling scale‑up from a 2 L working volume to 4 L without compromising cell growth, morphology, or virus yield (TCID50). Cytodex™ Gamma microcarriers delivered comparable performance while significantly reducing preparation time, contamination risk, and unit operations.
Introduction
Introduction
Cells used for virus propagation are sensitive and easily damaged, for example, by shear stress during cell expansion during bioprocessing. To minimize shear stress while supporting increased cell density, virus production is performed in a bioreactor with capacity to aerate increasing cell densities without increasing shear forces.
The WAVE™ 25 bioreactor lets you select different rocking motions for cell incubation. This allows for control of the shear stress and optimization of growth conditions for a variety of cell lines, such as robust cell lines with a high oxygen demand or more delicate cells.
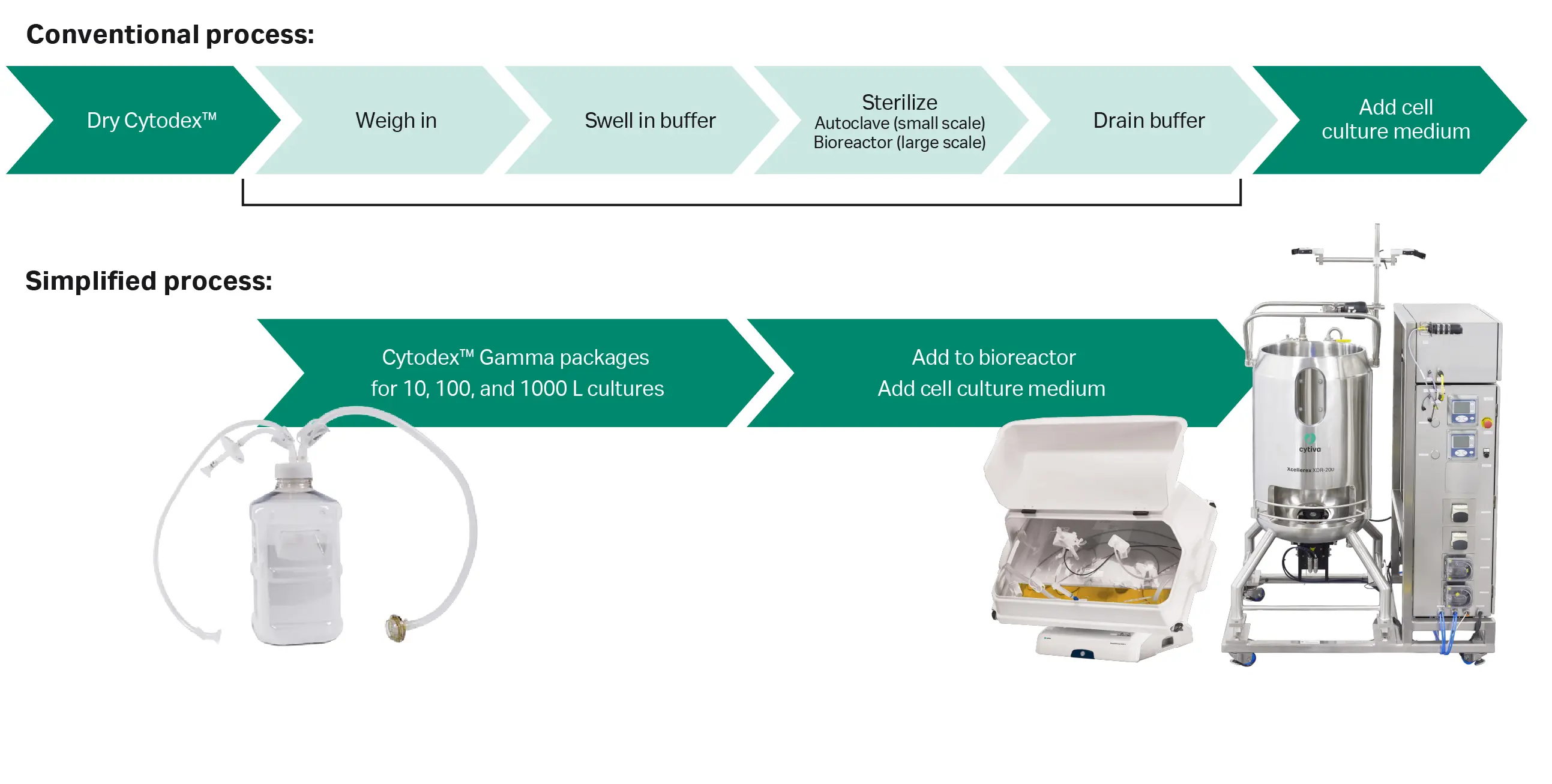
Vero cells are anchorage-dependent and can only proliferate when provided with a suitable surface. In bioreactor cultures, microcarriers are used to meet this requirement. However, Cytodex™ microcarriers need to be washed, sterilized, and equilibrated before use. For single-use bioreactors, microcarrier preparation is usually conducted in a separate reusable tank. Presterilized Cytodex™ Gamma microcarriers, on the other hand, are supplied in containers with flexible connection options for various cell culture vessels. This allows for the addition of the microcarriers directly to the production vessel without prior preparation (Fig 1). This not only reduces the time and workload, reducing the number of unit operations, but also lowers the contamination risk by allowing aseptic transfer from the container to the culture vessel.
Earlier work with the WAVE Bioreactor™ 20/50 showed a maximum usable working volume of 2 L without inducing critical shear stress. Here, we evaluated whether smoother rocking motion in the WAVE™ 25 allows an increase to 4 L. We also compared performance between Cytodex™ 3 and Cytodex™ 3 Gamma microcarriers.
Fig 1. Ready-to-use Cytodex™ Gamma microcarriers packaged in a single-use container simplify microcarrier preparation and transfer to various single-use bioreactor systems. Here, we compare the conventional multistep process using Cytodex™ microcarriers with a simplified process using Cytodex™ Gamma microcarriers.
Materials and methods
Materials and methods
Cell line and maintenance cultures
- We seeded thawed Vero cells (ECACC) into serum-free cell culture media in T-flasks before culture in Cell Factory systems.
- Cell culture: 37°C, 5% CO2 environment.
- For maintenance cultivation, we washed the cells with phosphate-buffered saline (PBS).
- Cell detachment: Recombinant protease using TrypLE Select.
- Seeding cell density: 4 to 5 × 104 cells/cm2.
- Before adding the cells to the bioreactor with microcarriers: Trypsin inhibitor added to the cell suspension, medium supplemented with 0.2% poloxamer 188.
Preparation of Cytodex™ 3 microcarriers
- Microcarriers weighed into siliconized glass bottles.
- PBS added (50–100 mL/g of microcarriers).
- Swelling of microcarriers for 3 h during which they were repeatedly mixed.
- For sterilization, we washed the microcarriers twice with PBS before autoclaving at 121°C for 20 min and washed twice with culture medium (30–50 mL/g) before use.
Preparation of Cytodex™ Gamma microcarriers
- Cellbag™ biocontainer inflated with air.
- Microcarriers added to Cellbag™ biocontainer: To make the transfer under a closed system, we used one of the connectors of the microcarriers container system and transferred directly to the bioreactor.
- Serum-free medium added.
- Mixture equilibrated before cell inoculation: 2 h at 37°C, 5% CO2.
WAVE™ 25 bioreactor cultures
- Two 10 L Cellbag™ bioreactors installed in a ReadyToProcess WAVE™ 25 rocker
- Connected to a ReadyToProcess™ CBCU controller.
- One of the 10 L Cellbag™ bioreactors was inflated with air and the serum-free medium and Cytodex™ 3 microcarriers were transferred to the bioreactor.
- Equilibration: 37°C and 5% CO2.
- For the second 10 L Cellbag™ bioreactor: Cytodex™ 3 Gamma microcarriers and medium added as described in the section Preparation of Cytodex™ Gamma microcarriers.
- Offset pH calibration on both culture bags was performed before cell inoculation.
- Inoculation: 0.2 × 106 cells/mL, 4 L working volume, pH 7.1, 37°C, 5% CO2.
- Rocking parameters:
- Rocking motion: 100% or 30%
- Rocking speed: 8 rpm at 6° angle increasing to 9 rpm when cells reached confluence.
- Sampling:
- Before sampling, rocking speed increased to 20 rpm for 1 min to ensure a homogeneous solution.
- Two hours after seeding, to ensure that the cells had attached to the microcarriers.
- Sampling every 24 h to determine cell density and morphology.
- Medium exchange: 50% of the working volume after 48 or 72 h.
- Cell infection at a cell density of 1 × 106 cells/mL.
Virus propagation
- Virus: Influenza A/Solomon Islands/3/2006/IVR145 (H1N1)
- Amount of virus particle (titer) calculated according to multiplicity of infection (MOI) for the virus strain.
- Prior to infection: ~ 50% medium removed.
- Virus maintenance medium added (serum‑free + trypsin + virus).
- Temperature reduced to 33°C.
- After 1 h: Working volume restored to 4 L.
- Harvest: Day 4, followed by TCID50 analysis in 96‑well plates.
Results
Results
Effect of rocking motion on cell culture performance
The WAVE™ 25 bioreactor system retains the simplicity of a single‑use rocking platform while adding automation and ergonomic features (Fig 2). The tilt function simplifies medium exchange and minimizes microcarrier loss.
Fig 2. (A) The ergonomic design of the WAVE™ 25 rocker makes activities such as sampling and harvest convenient and easy. (B) The rocker system in tilt position.
Our previous studies in the WAVE Bioreactor™ 20/50 system showed that the maximum working volume without introducing critical shear stress was 2 L, in a 10 L Cellbag™ bioreactor. We tested whether we could scale up the virus production process while keeping shear stress to a minimum. Thanks to the different rocking motion settings in the WAVE™ 25 bioreactor, we increased the working volume to 4 L by using a smoother rocking motion. Our findings were:
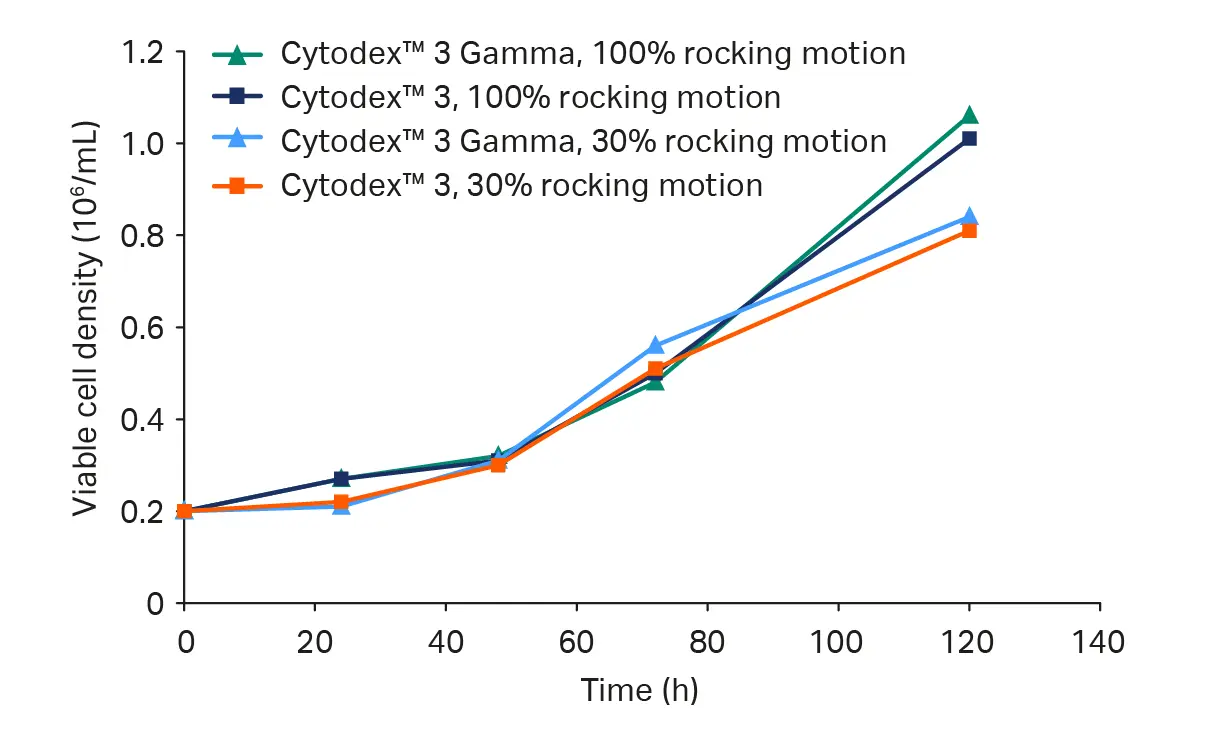
- Under both rocking motion settings (30% and 100%), cell growth was comparable through 72 h (Fig 3).
- At later time points and as cell density increased, 100% rocking motion provided improved cell growth, likely due to smoother acceleration and reduced shear as rocking speed slowed at the turning points.
- Cytodex™ Gamma microcarriers and Cytodex™ microcarriers supported equivalent cell growth dynamics (Fig 3).
Cell morphology
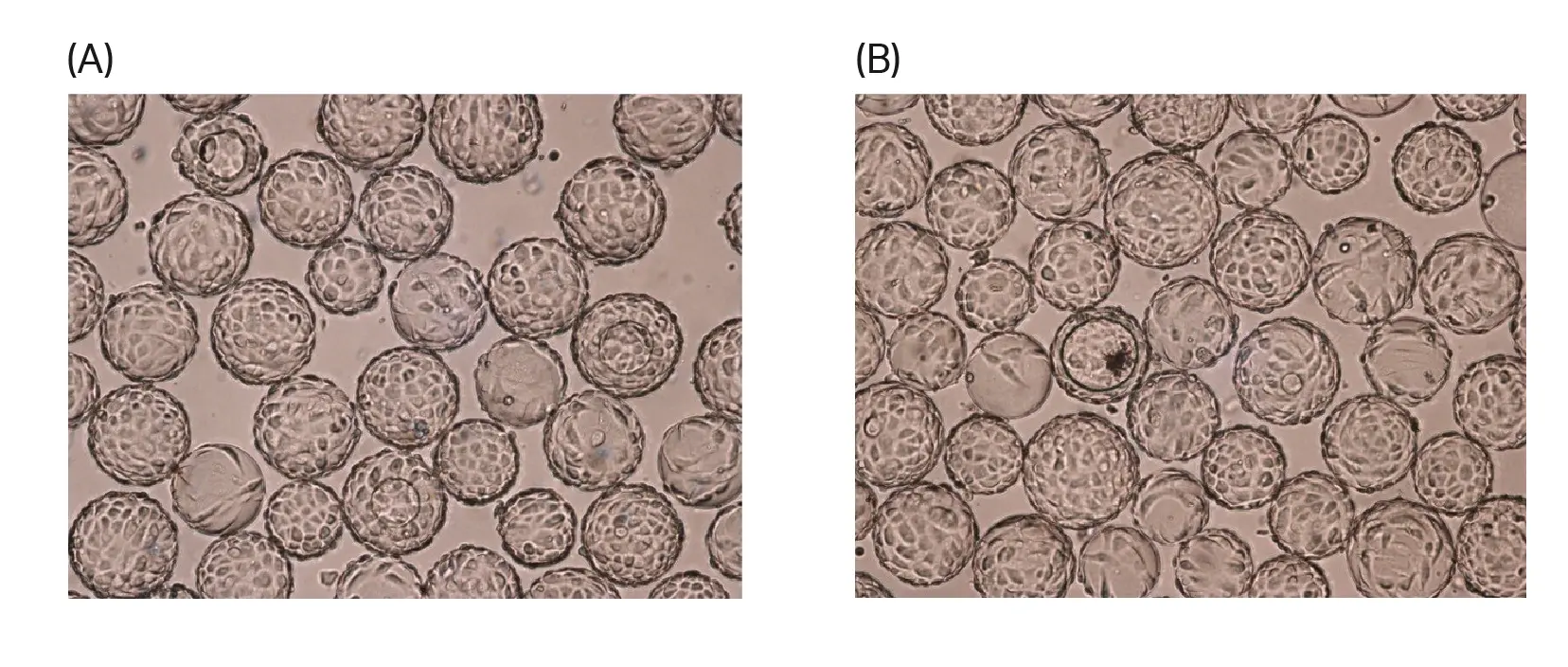
We evaluated the morphology of cells grown on either Cytodex™ Gamma or Cytodex™ microcarriers (Fig 4 to 7) using either a 30% or 100% rocking motion setting at the time of infection (96 h after inoculation) and at the time of harvest (96 h after infection). Cell morphology was comparable across all conditions.
Viable cell density and virus yield
We observed no difference in cell growth or distribution between the cultures (Fig 3). Virus yields were similar across all microcarrier and rocking configurations (Table 1).
Fig 3. (A) Viable cell density in cultures using either Cytodex™ 3 Gamma or Cytodex™ 3 microcarriers in 4 L medium. Cells were cultured in the WAVE™ 25 bioreactor using a rocking motion setting of either 30% or 100%.
Table 1. TCID50 analysis results
| Sample | TCID50 |
|---|---|
| Cytodex™ 3 microcarrier, 100% rocking motion | 107.1/mL |
| Cytodex™ 3 Gamma microcarrier,100% rocking motion | 107.5/mL |
| Cytodex™ 3 microcarrier, 30% rocking motion | 107.5/mL |
| Cytodex™ 3 Gamma microcarrier, 30% rocking motion | 107.7/mL |
Fig 4. Morphology of Vero cells grown on (A) Cytodex™ 3 Gamma or (B) Cytodex™ 3 microcarriers 96 h after inoculation. Bioreactor culturing was conducted using a rocking motion setting of 100%.
Fig 5. Morphology of Vero cells grown on (A) Cytodex™ 3 Gamma or (B) Cytodex™ 3 microcarriers 96 h after inoculation. Bioreactor culturing was conducted using a rocking motion setting of 30%.
Fig 6. Cytopathic effect on Vero cells grown on (A) Cytodex™ 3 Gamma or (B) Cytodex™ 3 microcarriers 96 h after infection. Bioreactor culturing was conducted using a rocking motion setting of 100%.
Fig 7. Cytopathic effect on Vero cells grown on (A) Cytodex™ 3 Gamma or (B) Cytodex™ 3 microcarriers 96 h after infection. Bioreactor culturing was conducted using a rocking motion setting of 30%.
Conclusion
This study demonstrates that the WAVE™ 25 bioreactor enables efficient influenza virus production from Vero cells using microcarrier cultures at double the previously validated working volume (4 vs 2 L). A 100%, smooth rocking motion minimized shear stress, which is critical for bioprocess scale-up, and enhanced late‑stage cell growth.
Cytodex™ 3 Gamma microcarriers performed equally well compared to standard Cytodex™ 3 microcarriers in terms of:
- Cell growth
- Morphology
- Virus yield (TCID50)
However, because Cytodex™ Gamma microcarriers require no preparation, they:
- Eliminate washing, sterilization, and equilibration steps.
- Reduce labor and unit operations.
- Minimize contamination risk through closed transfers.
Overall, the combination of the WAVE™ 25 bioreactor and Cytodex™ Gamma microcarriers provides a streamlined, scalable, and efficient process for influenza virus production using anchorage‑dependent Vero cells.
FAQ on the article content
FAQ on the article content
What is the main advantage of using the WAVE™ 25 bioreactor for influenza virus production?
The ReadyToProcess WAVE™ 25 bioreactor provides controlled, low‑shear rocking motion and scalable single‑use operation. Its smoother 100% rocking setting minimized shear stress and enabled an increase in working volume from 2 L to 4 L without compromising Vero cell growth or virus yield.
How do Cytodex™ 3 and Cytodex™ 3 Gamma microcarriers compare in performance?
Both microcarrier types supported equivalent Vero cell growth, morphology, and influenza virus production. TCID50 values were similar across all conditions, confirming comparable process performance.
Why choose Cytodex™ Gamma microcarriers over traditional Cytodex™ microcarriers?
Cytodex™ Gamma microcarriers are presterilized and ready to use, eliminating washing, sterilization, and equilibration steps. This reduces labor, streamlines single‑use workflows, and lowers contamination risk through closed, aseptic transfers.
Does smoother rocking motion reduce shear stress in microcarrier cultures?
Yes. The 100% rocking motion setting slows movement at turning points, creating smoother transitions and reduced instantaneous shear. This was particularly beneficial at higher cell densities in 4 L cultures.
What working volume can be achieved without increasing shear stress?
Using the WAVE™ 25 system with 100% rocking motion, the working volume for microcarrier‑based Vero cultures was increased from 2 L (legacy WAVE Bioreactor™ 20/50) to 4 L while maintaining low shear conditions.
Is virus yield affected by the type of microcarrier or rocking motion?
No. TCID50 analysis showed similar influenza virus titers regardless of microcarrier type (Cytodex™ 3 or Cytodex™ 3 Gamma) or rocking motion setting (30% or 100%).
What virus strain was used in this process?
The process used Influenza A/Solomon Islands/3/2006/IVR145 (H1N1), with infection parameters based on MOI calculations specific to the strain.
Are Cytodex™ Gamma microcarriers compatible with closed single‑use systems?
Yes. Cytodex™ Gamma is supplied in gamma‑irradiated, closed single‑use containers with flexible connection options, enabling fully closed and aseptic transfer to bioreactors.
Related links
Related links
ReadyToProcess WAVE™ 25 bioreactor
ReadyToProcess™ CBCU controller
Cellbag™ 10 L bioreactor container
CY13813