Bioreactors are widely used to cultivate cells during the development and manufacturing of modern biopharmaceuticals. Cells are very sensitive to changes in the culture environmental conditions, such as aeration, agitation, nutrients, and pH. This article discusses the importance of aeration and available options to control the oxygen mass transfer coefficient (kLa) within a bioreactor.
Delivering oxygen to cells
In cell culture, oxygen is a key substrate for growth, production, and maintenance activities. Cells obtain their oxygen in free and noncompound forms, called dissolved oxygen (DO). One of the most important functions of bioreactors is providing dissolved oxygen to cells continuously through a process called aeration.
Aeration in the bioreactor typically occurs when:
- Oxygen diffuses through overlay to the cell culture medium interface.
- Oxygen from the spargers dissolves in the cell culture through convection with the help of agitation.
Agitation disperses the oxygen bubbles and promotes mass transfer of the gas bubbles through the gas‑liquid (cell culture medium) interface. The rate of oxygen transfer (OTR) from gas to liquid interface is a function of physicochemical properties of the cell culture medium, the geometrical parameters of the bioreactor, and presence of cells.
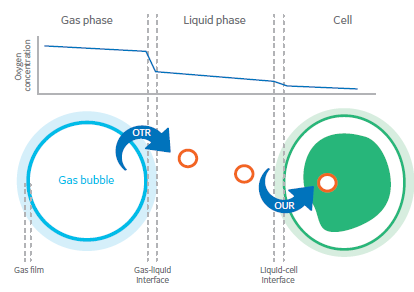
Fig. 1 Diagram of a gas bubble in liquid, showing how the bubble is released, solubilized, and transferred to a cell.
Oxygen utilization rate (OUR) is often cell line‑dependent. The following table lists the rates for common industrial cell lines (1, 2).
Oxygen utilization rates of cell lines typically used in biomanufacturing
| Cell line | OUR [10-13mol/cell/h] |
| DG44 (CHO) | 2 |
| CHO | 5.0–8.04 |
| NSO (myeloma) | 2.19–4.06 |
| MAK (hybridoma) | 4.16 |
| FS-4 (human diploid cells) | 0.5 |
| HFN7.1 (hybridoma) | 2 |
Due to its low solubility in liquid phase and increasing metabolic consumption by the cells with time, oxygen is supplied continuously to the cell culture. Oxygen supply is carefully controlled for optimal cell growth by manipulating bioreactor parameters.
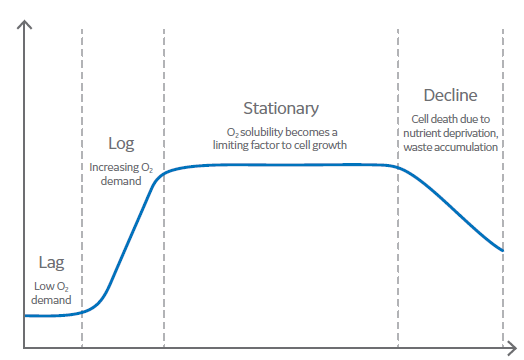
During batch cell culture, OUR (or OTR) is initially low during the lag phase, where cells are self‑synthesizing and there is little gain of cell density. As cell density increases during the exponential phase, OUR increases until OTR becomes a limiting rate, as determined by the mass transfer of oxygen into the bulk liquid.
Fig 2. Phases of cell growth.
The OTR and OUR rates are correlated by the oxygen mass transfer coefficient, kLa. Therefore, the OTR, through its correlation to kLa, defines a theoretical maximum cell density that could be achieved in cell culture.
Utility of kLa values
Because of this association with cell density, kLa values are particularly useful in the following scenarios:
Scenario 1: Evaluating scalability within the same bioreactor platform
The conventional scale‑up of bioprocesses is based on physicochemical and geometric similarity. kLa is kept constant for this scenario. The OTR should remain constant for a bioreactor platform with geometric similarity (such as Xcellerex™ bioreactors). Bioreactor physical characteristics at the different scales are altered to provide the necessary OTR at controlled temperature, pH, and DO to achieve the target cell density.
Scenario 2: Technical transfer across different bioreactor designs
During the comparison, kLa is utilized as a target performance metric when a process is transferred from one bioreactor platform to another design. Bioreactor hardware design (e.g., stirrer geometry and aeration‑sparger option) and running parameters (e.g., gas flow rate or power input) are altered to achieve a similar kLa, providing a similar cell density.
Equation for kLa
Imagine a gas bubble in liquid. For this discussion the gas bubble contains oxygen, and the liquid is the liquid in a bioreactor. kLa can be represented by the following equation:
kLa = kL × a
- Where kLa is the mass transfer coefficient from the gas to liquid phase, given in sec‑1
- kL = liquid side mass transfer coefficient (resistance in gas side film can be neglected)
- a = bubble surface (available for diffusion)
Key variables that impact kLa values
Any change to process and engineering parameters or to physical characteristics will have an impact on kLa and should be considered when evaluating bioreactor platforms and performing scaling calculations.
Here are four key variables that can affect kLa values:
1. Gas bubble size
When gas bubble size decreases, surface area and gas residency time increases, causing bubbles to stay in the culture longer. Thus, there is a greater opportunity for oxygen to release mass transfer into the cell culture medium. An increase in this oxygen residence time improves kLa.
2. Mixing
In a bioreactor, mixing is used to eliminate gradients of concentration (cell, gas, medium, and nutrient), temperature, and other properties. Mixing time is widely used to characterize mixing efficiency in a bioreactor. Mixing efficiency is one of the most significant factors affecting both performance and scale‑up in a bioreactor.
Gas bubble size and residency time are highly dependent upon three mixing conditions: impeller type, speed, and location(s). kLa values generally increase as tip speed increases. However, tip speed is proportional to shear forces that can lead to cell death. Bioreactors, therefore, are designed with different impeller types, combinations, and locations to achieve target kLa values without creating these shear forces.
Generally, kLa values are closely associated with impeller design, with Rushton typically higher than paddle, which is typically higher than marine and pitched impeller.
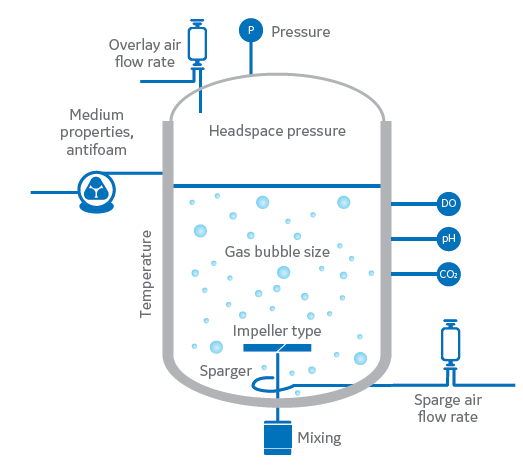
Fig 3. Diagram of a bioreactor process showing key factors that can influence kLa values.
3. Air flow rate
Higher oxygen availability drives kLa increases. Increasing oxygen supply to a bioreactor drives this availability and can be controlled by modifying concentration (air vs O2 enrichment) and volumetric flow. Although high kLa values are desirable, it is important to consider the actual operating conditions and implications to cell viability and associated process costs.
For example, high air flow rates can cause cell damage due to shear forces. Excessive foam might also be generated, requiring a high concentration of antifoam that could hinder downstream processing. Additionally, higher air flow rates require a larger exhaust filter area, driving consumable cost increases.
4. Properties of the liquid or medium
During cell culture, small bubbles collide and coalesce to form larger bubbles, decreasing surface area (a) and subsequently kLa. Be aware of reported kLa values in which high salt concentrations are used, because this can prevent bubble coalescing. Antifoaming agents are used to influence surface tension, resulting in reduced bubble coalescence and foaming.
However, this principle does not always lead to increases in OTR wherein antifoam also reduces bubble mobility, which subsequently reduces the kLa (3).
Other factors that affect cell culture kLa
5. Measurement method
Several different methods are used. Most commonly the nitrogen stripping (i.e., gassing‑out) method is employed.
When scaling a process within the same platform, it is important to use an identical method for measuring kLa. kLa, when combined with process engineering parameters (i.e., tip speed, power input), can be used to experimentally determine the cell density in a larger bioreactor compared with a smaller bioreactor.
6. Temperature
Increasing temperatures inversely affects both the volumetric mass transfer coefficient and oxygen solubility in culture medium. Oxygen solubility in pure water falls with increasing temperature (i.e., ‑0.5 × 10‑3 kg/m‑3 between 35°C and 30°C; 3).
Therefore, it is important to note the temperature conditions from vendor‑supplied characterization data.
7. Sparger characteristics
kLa values will vary widely with sparger characteristics, including number, pore size, and surface area, because these factors affect bubble size, gas velocity, and flow rates.
Conclusion
As discussed here, kLa is impacted by multiple factors. A thorough understanding of a bioreactor platform’s physical design, mixing mechanism, sparging options, as well as cell line characteristics will inform decision‑making when scaling cell culture processes up and out.
Learning and education
- Hands-on bioreactor course – CELL1
- Free online course – get to know bioreactors
- Free online course – host cells
- Browse our full online course catalog
Cytiva solutions
References
- Ruffieux PA, von Stockar U, Marison IW. Measurement of volumetric (OUR) and determination of specific (qO2) oxygen uptake rates in animal cell cultures. J Biotechnol. 1998;63(2):85-95. doi: 10.1016/s0168-1656(98)00046-7.
- Xiu Z-L, Deckwer W-D, Zeng A-P. Estimation of rates of oxygen uptake and carbon dioxide evolution of animal cell culture using material and energy balances. Cytotechnology. 1999;29(3):159-166. doi: 10.1023/A:1008004618163.
- Doran PM. Bioprocess Engineering Principles, Second edition. Waltham, MA; Elsevier Ltd: 2013.