Perfusion processes enable continuous operation over extended periods of time by constantly providing fresh nutrients for the cells and simultaneously removing spent media and waste products, as compared to batch and fed-batch processes. The key benefits of perfusion processes are compact process design, flexibility, increased productivity, increased yield, and consistent product quality.
The purpose of the described study is to demonstrate a connected mAb perfusion cell culture process using Xcellerex XDR 50 integrated with the Xcellerex Automated Perfusion System (APS). The process target was to achieve a cell concentration of 70 MVC/mL and a cell viability of greater than 90% for 10 days in a closed and connected process. In addition, the stretch goal was for the cell harvest to deliver a daily average IgG titer of 0.72 g/L to the harvest hold bag and maintain a Cell Specific Perfusion Rate (CSPR) of 20 pL/cell/day.
Connected perfusion process dynamics
There are many methods for operating perfusion cell cultures in order to retain cells and product in the culture. These techniques are either based on size or density of the target cell or protein for separation. Devices like continuous centrifuges, alternating tangential flow filtration, spin filters, gravity settlers, and acoustic wave separation are used as cell retention devices. Each technique has associated pros and cons to it, however, choosing the right system is key.
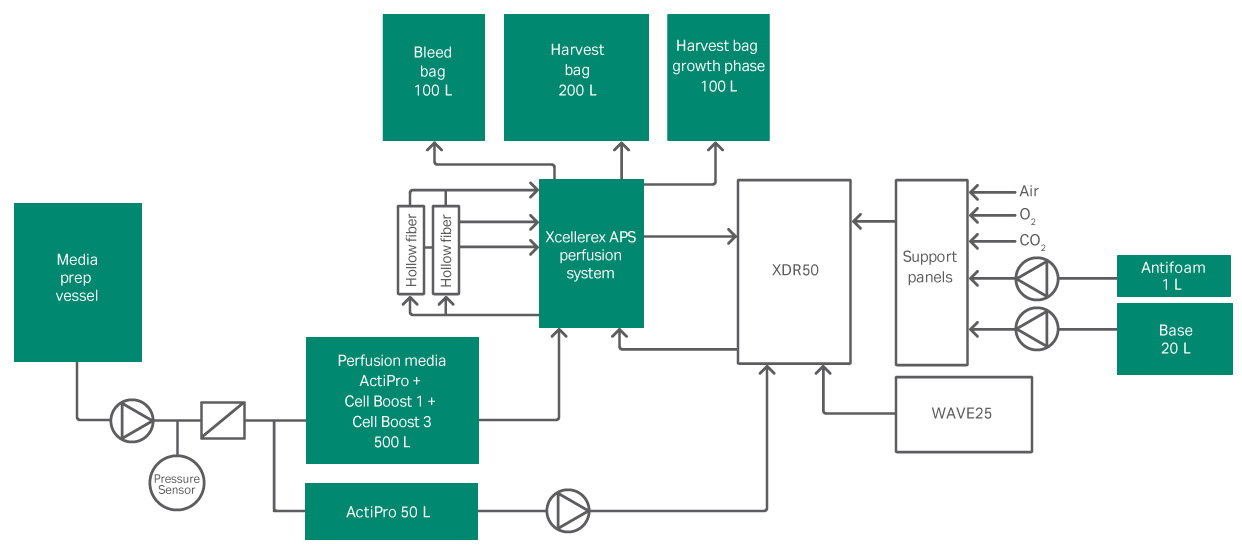
In this study, Xcellerex APS utilizes microfilters (MF) as a cell retention method. The proposed tangential flow filtration (TFF) based system consists of a low shear pump to recirculate process fluid through hollow fiber filters and back to the bioreactor. Pumps for perfusion media feed addition, permeate harvest, and cell bleed are regulated by feed-back controllers. Single-use pressure sensors enable continuous online monitoring of transmembrane pressure (TMP) and delta P , to predict filter performance. Flow sensors and weight scales are used for tight control of shear rate, fresh media addition, and spent media removal. This system provides benefits like easy and flexible setup, short residence time of process fluid in the recirculation loop, automated HF filter switch to a backup filter, and tight and accurate perfusion control with added automation. To improve efficiencies further, the scope of this project was to conduct the entire process in a connected single-use 50 liter manufacturing line.
Materials and methods
Prior to this study, a proof of concept study was performed to develop and optimize the operating parameters. More information on the small-scale study can be found here.
A recombinant Chinese Hamster Ovary (CHO) K1 cell line producing an IgG1 antibody under the glutamine‐synthetase (GS) selection system was used throughout this study. In the initial batch phase, the cells were recovered from cryopreservation and expanded using shake flasks in HyClone ActiPro basal medium. The final seed expansion to 10 liters was conducted in a WAVE 25 bioreactor. The operation parameters can be seen in Table 1.
For the perfusion operation, HyClone ActiPro basal medium supplemented with HyClone Cell Boost 1 and HyClone Cell Boost 3 was chosen. Extensive media optimization studies were performed on a variety of media and feed combinations to determine the ideal conditions for steady state perfusion with the CHO K1 cell line. More details of these studies can be viewed here. The combination of ActiPro and Cell Boost 1 and 3 were optimal for this particular cell line but we would recommend our Cell culture media development service teams to assist in identification of the optimal combination for specific cell lines.
Once a set cell concentration was reached, the perfusion process was initiated. The perfusion process was conducted using the connected set up shown in Figure 1. The operating parameters for the perfusion operation can be viewed in Table 2. Clarification of the cell harvest and collection of the product was performed continuously through the Xcellerex APS using the parameters in Table 3. The product was collected in an XDM 200 hold tank for further downstream processing.
Table 1. Operating parameters of the WAVE 25 inoculum bioreactor
| Parameter |
Setting/comment |
| Medium | ActiPro |
| Reactor type | Cellbag 20L with ReadyToProcess WAVE 25 |
| Rocking | 22 to 25 rpm 6° rocking angle Standard rocking profile |
| Gas flows | 0.25 LPM |
| Seed cell concentration | 0.7 × 106 viable cells/mL |
| pH set point | Low volume stage: No pH control (initial 7.5% CO2 in headspace) Full volume stage: pH controlled at 7.0 (no base) |
| DO set point | 40% (with drift from 100%) |
| Working volume | 2 L for first 2 days, then 5 L, and finally 10 L |
| Target cell concentration target at transfer | 6 × 106 viable cells/mL |
| Viability criteria at transfer | > 95% |
Table 2. Operating parameters of the production stage bioreactor
| Parameter |
Setting |
| Culture working volume | 50 L |
| Perfusion strategy | Start perfusion when cells have reached 2-4 MVC/mL Keep a perfusion rate of 20 pL/cells/day. Maintain steady-state at 70 MVC/mL by cell bleeding for 10 days |
| Temperature setpoint | 37°C |
| pH setpoint | 7.0 (Day 0) 6.8 (Day 1 and onwards) |
| DO setpoint | 40% |
| Target inoculation cell concentration | 1 MVC/mL |
| Do sparge rates | Day 0 to approx. Day 4 (ca 8 MVC/mL): 20 µm sparger, air on demand Day 4 and onwards: 20 µm sparger, additional 100% oxygen on demand |
| CO2 stripping strategy | start at 1 SLPM. Increase by steps of 0.5 SLPM to keep pCO2 below 20 kPa |
| Target viable cell concentration | 70 MVC/mL (controlled-state) |
| Target viability | > 90% |
| Culture duration | 10 days at controlled-state |
| Culture medium | Expansion batch media: ActiPro Perfusion media: ActiPro, 13.94% Cell Boost 1 (10% w/v stock solution), 15.80% Cell Boost 3 (5% w/v stock solution) |
Table 3. Operating parameters of the Xcellerex APS
| Perfusion process run parameters |
Set point |
| Flow path conditioning flow rate | 5 LPM |
| Perfusion recirculation flow rate | 6 LPM |
| Shear rate | 2000 ±100 s-1 |
| CSPR rate | 20 pL/cell/day |
| Bleed rate | Based on biomass sensor (mLPM) |
| TMP for filter switch | 0.8 bar |
Fig 1. Experimental set-up using Xcellerex XDR 50 and Xcellerex APS.
Results
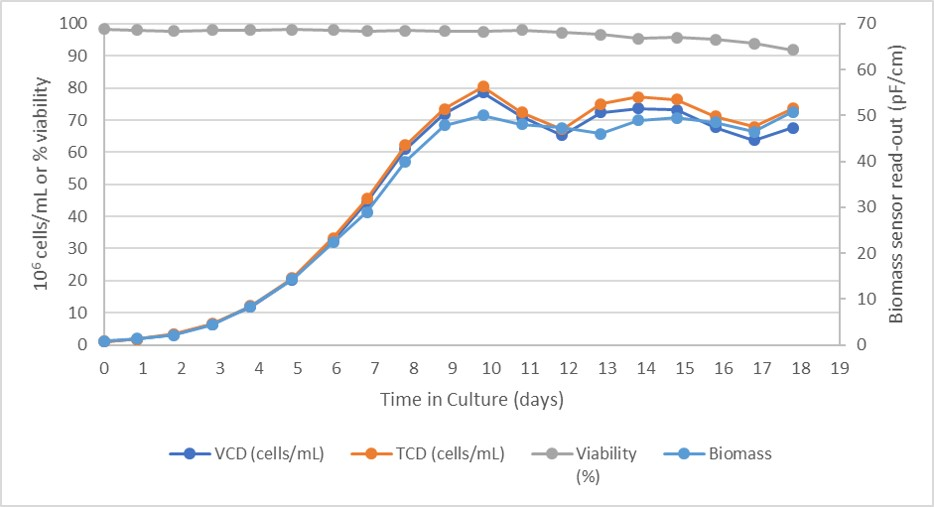
The batch was inoculated with a seed density of 1.0 × 106 cells/mL to adapt the cells to a new environment, reduce the lag phase, and allow an early start of the perfusion phase. The culture had an initial rapid growth phase for the first five days with a population doubling time (PDT) averaging 30 hours followed by a slower growth phase before reaching the controlled state at day 7 (Fig 2).
On day 7, the steady state process was started by continuous cell bleed based on the growth rate, reactor volume, and VCD value. The cell bleeding was automatically controlled by the Xcellerex APS. The perfusion control strategy was based on a constant XDR weight with ± 200 g accuracy range. In this strategy, perfusion media addition was tightly controlled, accurate, and predictable, whereas permeate harvest was controlled to maintain a steady XDR weight and process. From the pre-study it was known that it was not possible with one or two daily off-line sample data point to establish a controlled state. The Xcellerex XDR 50 in-line biomass sensor provided the necessary data for controlling the bleed rate and keeping the bioreactor in a controlled state.
Fig 2.Cell growth and viability in Xcellerex XDR 50 bioreactor.
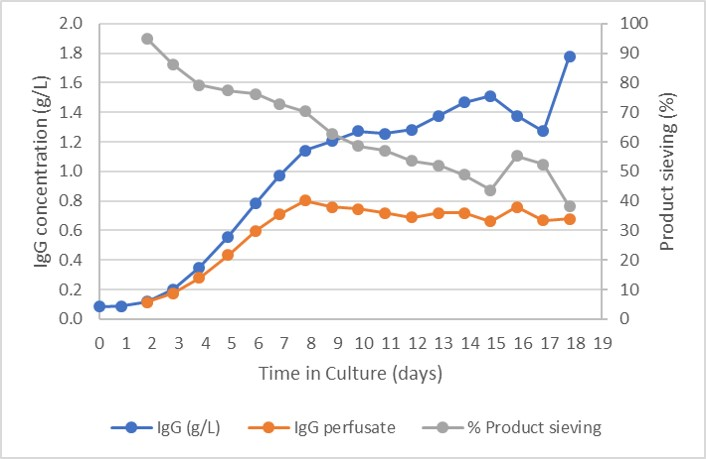
Product concentration is shown in Figure 3 with product concentration in the bioreactor as well as in the perfusate. The Figure shows that the product accumulates in the bioreactor over time due to product retention. The product concentration during the exponential growth phase was 668 mg/L and a peak product concentration of 985 mg/L was reached during the controlled state. The CSPR rate was maintained at 20 pL/cell/day.
Fig 3.Product concentration in bioreactor, perfusate, and the product sieving.
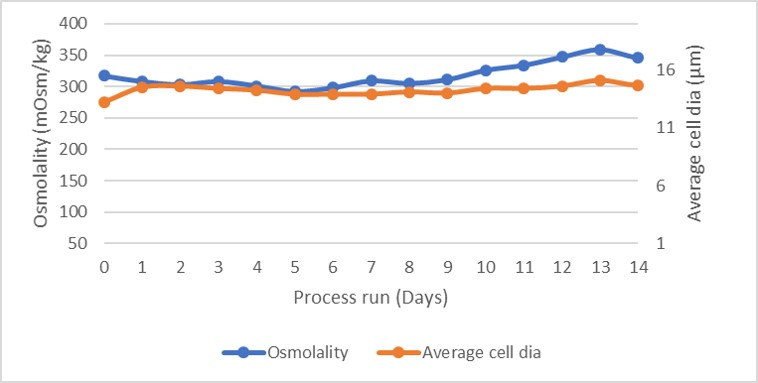
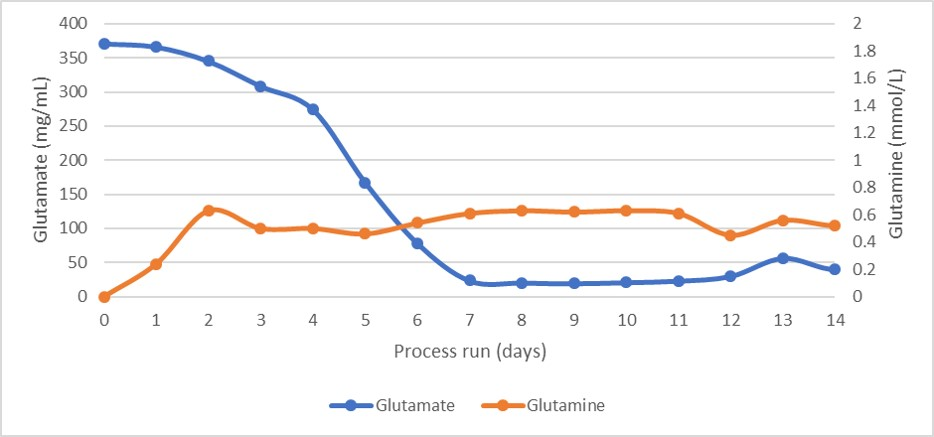
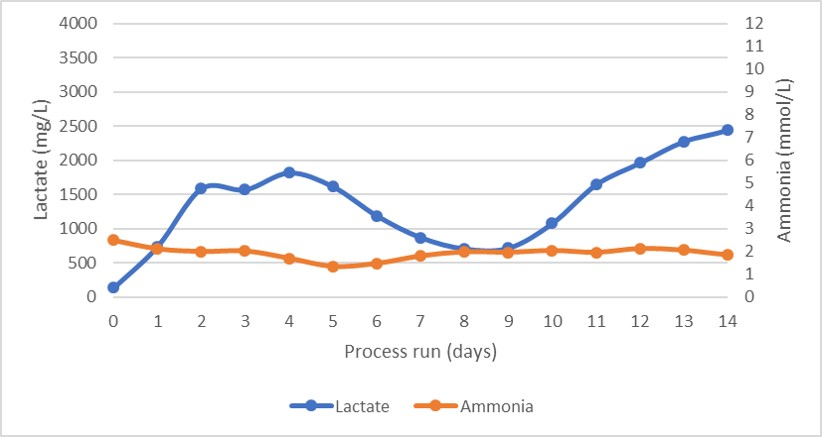
Osmolality and average cell diameter profiles of the cultures are shown in Figure 4. The osmolality profile was steady, suggesting a very well-balanced feed of nutrients and removal of waste products, as unconsumed nutrients would have increased osmolality. As shown by the glutamate and glutamine profiles in Figure 4 and the lactate and ammonia profiles in Figure 5.
Fig 4.Osmolality and average cell diameter profile.
Fig 5.Glutamate and glutamine concentration profiles during the perfusion cell culture.
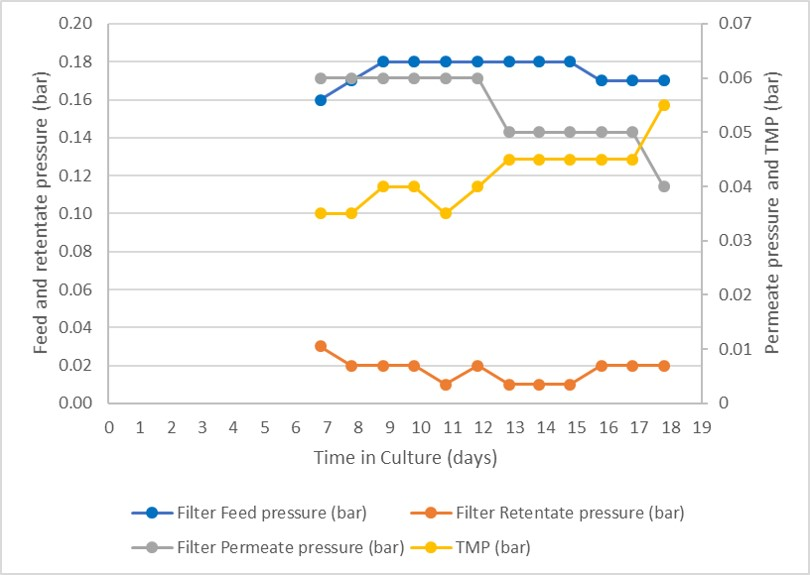
Feed, retentate, and permeate pressure were monitored during the process run, and the resulting TMP are reported in Figure 6. As can be seen, all the pressures remained constant throughout with the TMP only starting to rise after day 10. This demonstrates only limited filter fouling of the single filter that was used during the entire run.
Samples were tested for bioburden and no viable microorganisms were detected in the majority of the analyzed intermediates and endotoxin levels were below detection limit for all samples collected. For two of the samples, 1 CFU was reported, while duplicate samples were negative. Positive results were most likely attributed to sampling or testing errors and not confirmed with endotoxin results or a second sample.
Fig 6.Lactate and ammonia production profile
Fig 7.Pressure profile during the perfusion process.
Conclusion
The results of the study show that a continuous perfusion cell culture with a high cell density can be successfully cultivated in a connected process using the Xcellerex APS integrated with Xcellerex XDR 50 bioreactor system. In this study, we were able to successfully run the perfusion cell culture for 18 days with a cell viability greater than 90%. The perfusion cell culture was maintained in a controlled state at 70 MVC/mL for 10 of those days. In addition, the cell harvest delivered a daily average IgG titer of 0.72 g/L to the harvest hold bag and maintained a CSPR of 20 pL/cell/day.
In the presented run, the XDR APS perfusion system was integrated with XDR 50 bioreactor using a single-use (SU) flow path, XDR 50 bioreactor bag, and ReadyToProcess assemblies. The connected SU flow path eliminates cross contamination and reduces batch turn over time. All wetted parts can be conveniently disposed of after the process run. The system offers extensive monitoring and control capabilities through the Wonderware software.
References
- Mayrhofer, P., Reinhart, D., Castan, A. and Kunert, R. Rapid development of clone‐specific, high‐performing perfusion media from established feed supplements. Biotechnology Progress; 36(2).