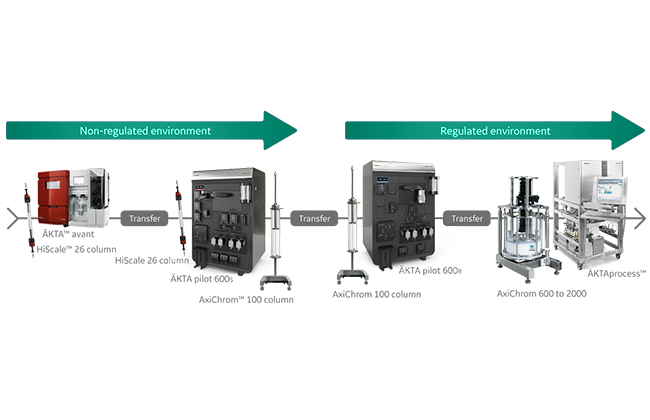
Be one step ahead in preparing for future GMP production
Transferring a process from a laboratory-scale idea to GMP-compliant commercial product is a resource-intensive step in biomanufacturing.
Let us help you find a way to make this transition less demanding.
When scaling, you can make better use of existing chromatography columns while avoiding overcapacity. Try this alternative approach to achieve a constant residence time.
Read moreKnowledge Article
Adapting single-use chromatography to manufacturing scale
While single-use technologies are widely adopted in upstream processes, what is required to move single-use technologies into downstream manufacturing scale?
Read more
How can you be one step ahead in process development and prepare for future GMP work? Learn what tech transfer experts say about this. And discover how to apply their approach to your process.
Read more