By Anna Ucher, University of Massachusetts
The recombinant adeno-associated virus vector (rAAV), for example, the rAAV5 serotype, has been widely evaluated as a gene therapy delivery system. In this article, we show how a HyClone™ peak expression cell culture medium promotes recombinant AAV production. We investigated the scalability of the process in HyClone™ peak expression medium in Xcellerex™ XDR-10 and XDR-200 bioreactors, and the performance was compared with the shake flask cultures. HyClone™ peak expression medium showed improved growth, viability, and rAAV productivity in HEK293.2 suspension cells.
Introduction
The rAAV vectors have been evaluated as gene-therapy delivery systems in preclinical and clinical studies for the correction of genetic diseases such as cystic fibrosis and hemophilia B (1). Several generations of rAAV have been developed to enhance safety and productivity and to produce more effective therapies (2). However, AAV5 serotype is common in clinical studies, and it makes a suitable model for the process development of rAAV.
The human embryonic kidney (HEK 293.2) suspension (sus) cell line supports the production of rAAV5 with triple plasmid transfection as a packaging cell line. Optimal cell performance, however, depends on several factors such as growth and productivity of the specific cell clone, the composition of the culture medium, and culture conditions such as agitation, aeration, and temperature. An understanding of how these factors influence metabolic processes is essential when designing cell culture media. Knowledge about a cell line’s metabolic activity, nutritional requirement, and waste creation helps to ensure that the correct combination and amounts of nutrients are used to minimize waste generation, which can result in cell toxicity.
Supporting transient transfection and cell growth
HyClone™ peak expression medium is a protein-free and animal-derived component-free medium that promotes recombinant AAV production. The medium was developed to support growth with high viable cell density (VCD), transient transfection for expression of a variety of recombinant AAVs, and productivity of HEK 293.2 sus cells in suspension cultures.
We evaluated cell growth, viability, and rAAV productivity of HEK 293.2 sus cells cultured in HyClone™ peak expression medium in shake flask cultures. Scalability of the process was investigated in Xcellerex™ XDR-10 and XDR-200 bioreactors using the modified medium and the performance was compared with the shake flask cultures.
Cell medium adaptation
HEK 293.2 sus cells (ATCC) were adapted to HyClone™ peak expression medium. Cell adaptation was performed by direct transfer cells grown in BalanCD™ HEK293 medium (Fujifilm Irvine Scientific) using a seed cell density of 5.0 × 105cells/mL. The cells were subcultured every 3 to 4 days (d). Subculturing of the cells was conducted by adding fresh medium at room temperature. Subcultivation of the cells was continued until cells reached population division time (PDT) between 24 to 30 h and with cell viability above 90%.
Shake flask cultures
The HEK293.2 sus cells were seeded into 30 to 50 mL of HyClone™ peak expression medium at a cell density of 0.4 ×106 cells/mL in 125 mL shake flasks. Culturing was conducted in a shaker incubator at 140 rpm, 37°C, and 5% CO2. Cultures were terminated when cell count and viability started to decline. The cell culturing was performed in triplicate runs.
Virus propagation
Cell cultures were transfected with three plasmids using a triple plasmid transfection system. The system consisted of a pHelper plasmid delivering adenoviral genes, a Rep/Cap (replication and capsid proteins) plasmid, and a third plasmid containing the gene of interest (GOI, in this case green fluorescent protein [GFP] was used for this study) between the inverted terminal repeat sequences (ITRs), which are essential for packaging it into AAV capsid. The ratio of helper:Rep/Cap:GOI plasmid was 2.0:1.5:1.0. Linear polyethyleneimine (PEI, Polysciences Inc.) was used to induce transfection at 2:1 (PEI to DNA ratio) in a culture of 2 × 106cells/mL density. Time of harvest (TOH) was performed 4 d post-transfection, and virus titer was measured by droplet digital PCR (ddPCR) against green fluorescent protein (GFP) transgene cassette.
Virus titer
About 900 µL of culture was treated with 100 µL of 10× lysis buffer (5% Tween™ 20, 10 mM magnesium chloride, 200 mM Tris, pH 8.0) and Turbonuclease enzyme at 20 U/mL for 4 h at 37°C in a shaking incubator. The aliquots were centrifuged at 15 000 × g for 10 min and the crude lysis supernatant was analyzed by ddPCR.
Bioreactor cultures
HEK 293.2 sus cells were seeded at a cell density of 0.5 × 106 cells/mL into HyClone™ peak expression medium in a single-use Xcellerex™ XDR-10 L cell culture bag in a final bioreactor batch volume of 9 L. Cells were cultured in the XDR-10 bioreactor system using culture parameters listed in Table 1. The cell culture was expanded from shake flasks through WAVE™ bioreactor (Xuri™ Cell Expansion System W25) and then into XDR-200 bioreactor.
Table 1. Bioreactor conditions for scale-up studies
| Parameter | XDR-10 bioreactor run | XDR-200 bioreactor run |
| Culture medium | HyClone™ peak expression medium | HyClone™ peak expression medium |
| Working volume | 9 L | 180 L |
| Impeller speed | 120 rpm (up-flow direction) | 158 rpm (up-flow direction) |
| Temperature | 37°C | 37°C |
| pH set point | 7.3 (controlled by CO2 and base) | 7.3 (controlled by CO2 and base) |
| Dissolved oxygen (DO) set point | 40% (controlled by oxygen enriched air) | 40% (controlled by oxygen enriched air) |
| O2 flow | Proportional integral derivative (PID) controlled | PID controlled |
| CO2 flow | PID controlled | PID controlled |
Results
Adaptation to HyClone™ peak expression medium
Clonally selected HEK 293 cells in BalanCD™ HEK293 medium (Fujifilm Irvine Scientific) were directly thawed in HyClone™ peak expression medium. After 3 to 4 d, the cells were split to 0.4 to 0.5 ×106 VC/mL and were maintained in culture for a few passages. VCD as well as PDT were monitored (Fig 1).
A cell bank was created at passage 5. Cells were frozen in the HyClone™ peak expression medium with 10% (dimethylsulfoxide) DMSO, concentrated to 20 × 106 VC/mL and 1 mL aliquots were transferred to cryovials. The cell bank was frozen in CoolCell™ at -80°C overnight and then transferred to a liquid nitrogen storage tank.
After thawing of multiple cell bank vials, we monitored VCD and population doubling time (PDT) for consistency (Fig 2).
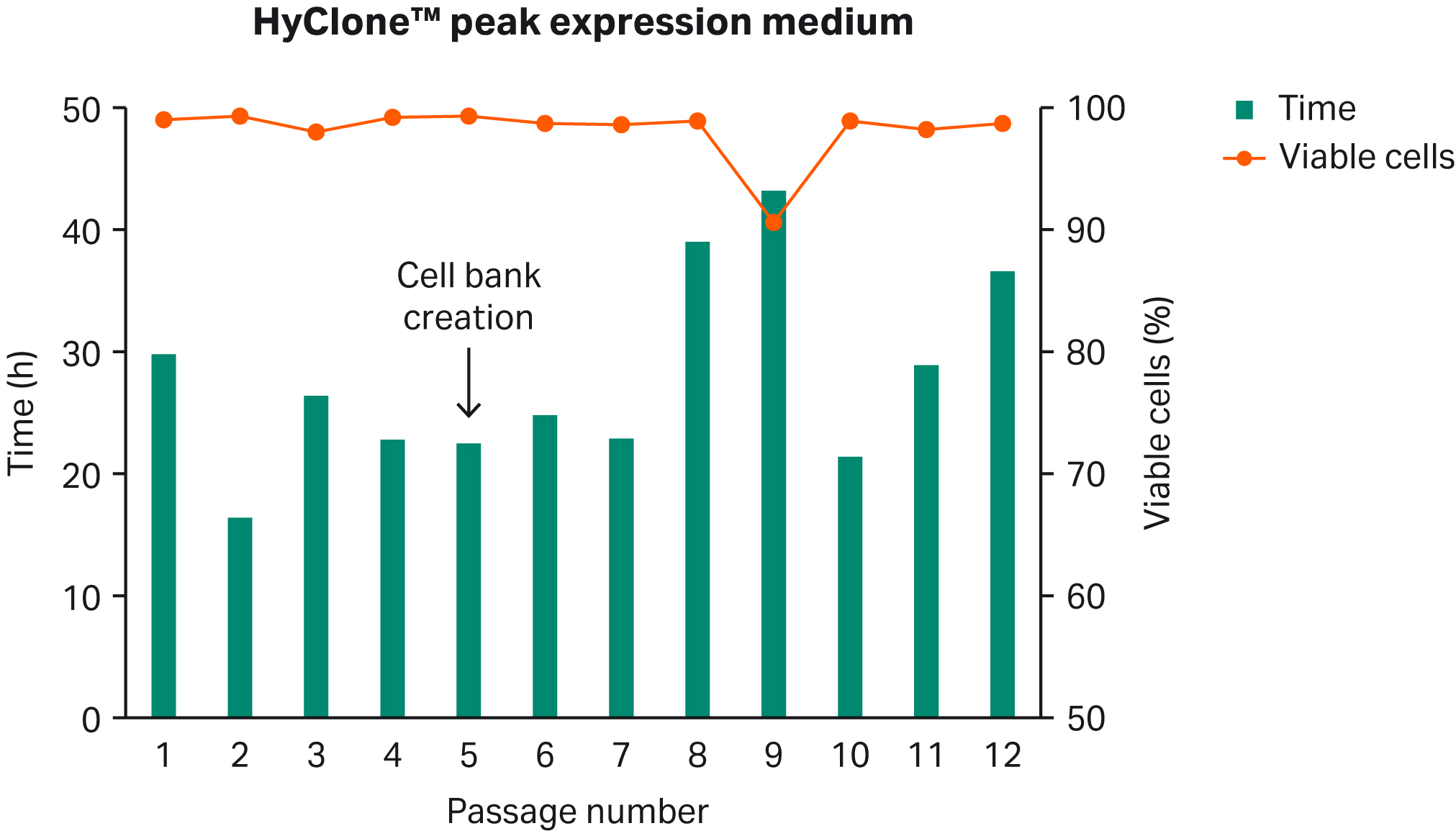
Fig 1. Direct HEK 293 cell adaptation to HyClone™ peak expression medium.
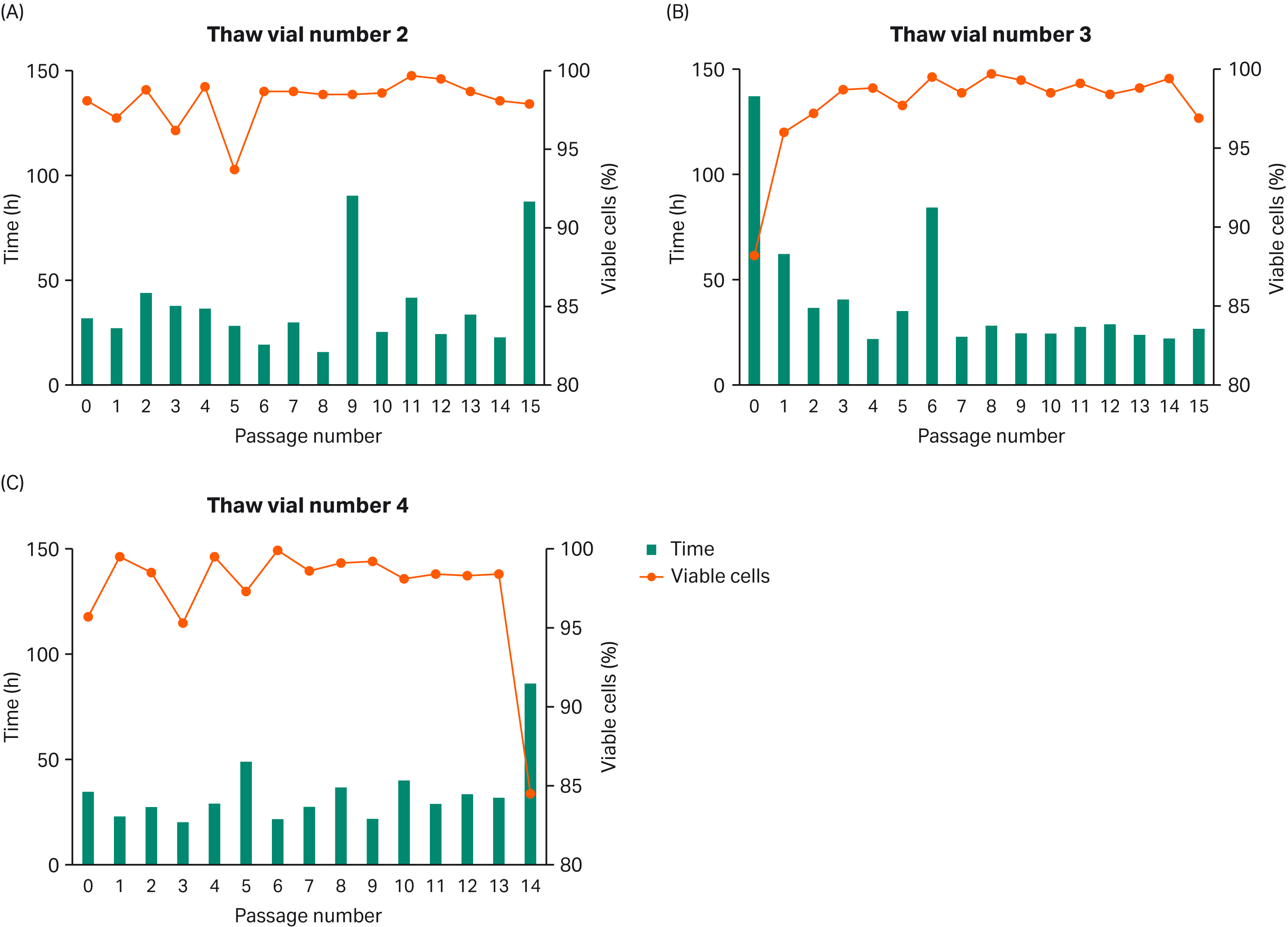
Fig 2. Multiple cell bank vials from passage 5 were thawed and VCD and PDT were monitored.
High-density culture in shake flasks
During this experiment, we cultured the cells at different passages in shake flasks using HyClone™ peak expression medium for a up to 13 d (Fig 3). VCD, cell viability, and PDT were monitored throughout. We can see from Figure 3 that the HyClone™ peak expression medium supports cell growth between 6.0 and 9.0 × 106 cells/mL.
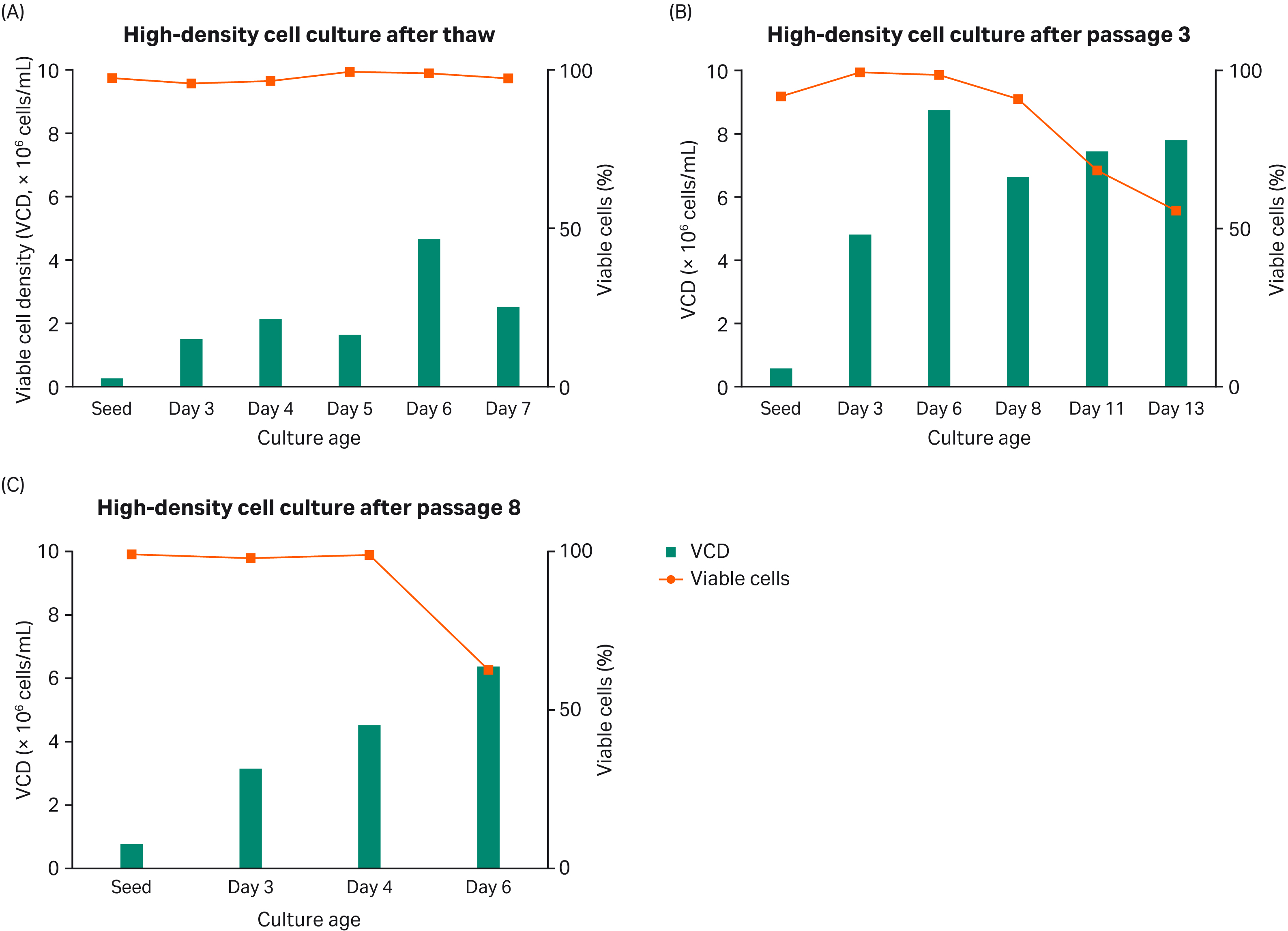
Fig 3. High-density cell culture in shake flasks (A), after thawing (B), after passage 3 and after passage 8 (C).
Transient transfection optimization in shake flasks
Transient transfection of adapted HEK293.2 suspended cells was performed using a triple plasmid transfection approach. In the procedure, linear polyethyleneimine (PEI) forms a complex with DNA, which is then introduced into the host cells. Triple transfection with 2 µg of DNA per 2 × 106/mL cells was used in the triple transfection of rAAV5-green fluorescent protein (GFP) to the HEK293.2 suspension cells in shake flasks.
To examine the effects of the culture medium on the stability of PEI-DNA complexes, HyClone™ peak expression medium was used in different complexing volumes, either 5% or 10% of the total rAAV production volume (Fig 4). Each condition was conducted in triplicate in shake flasks. The 10% volume was more consistent for repeated measurements and it is the recommended volume to use in rAAV production experiments for HEK293.2 suspension cells with HyClone™ peak expression medium.
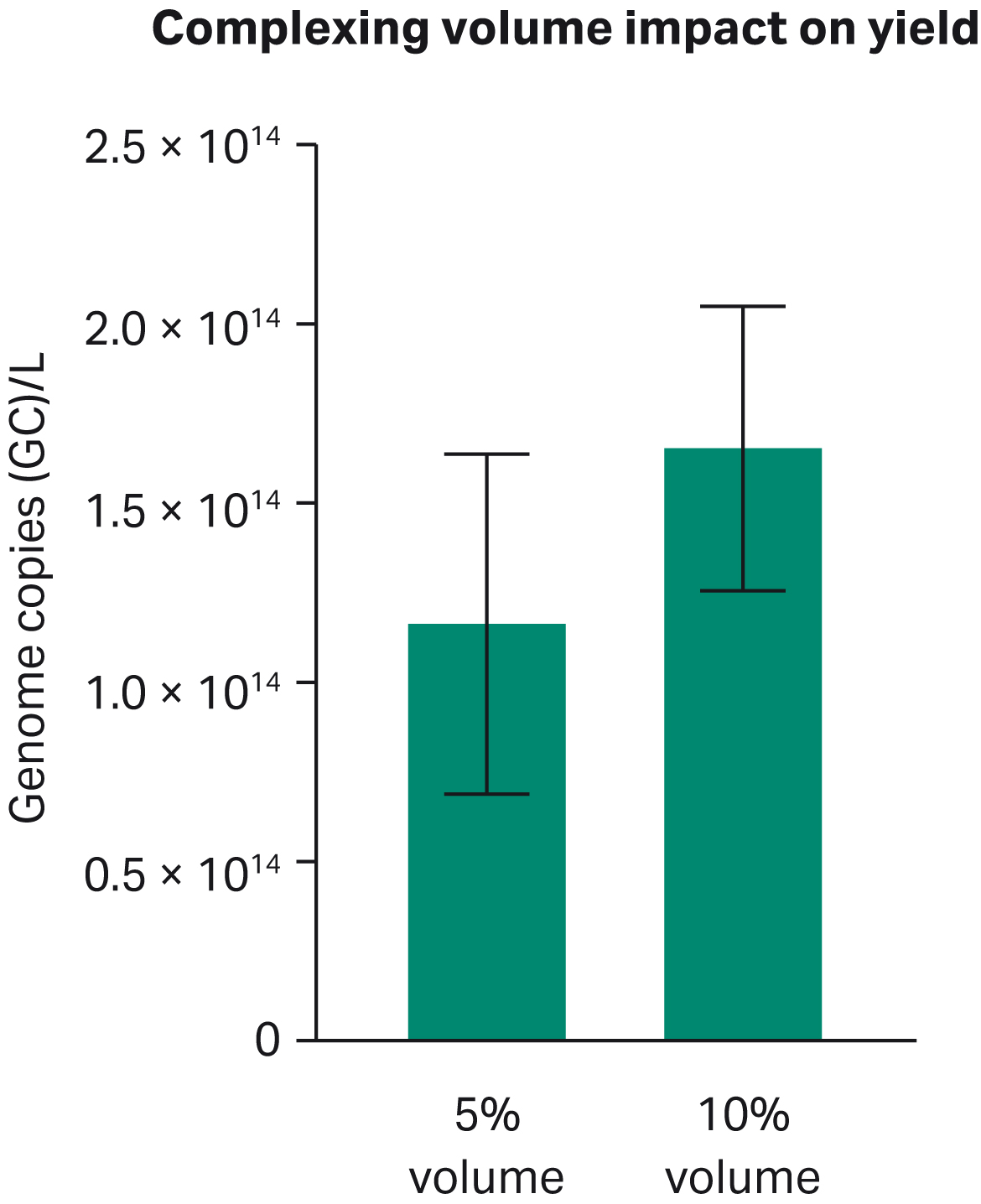
Fig 4. The difference in transfection yield on various volumes for complexing PEI and DNA was compared in 5% and 10% complexing volumes in shake flasks using HyClone™ peak expression medium. Genome copy number = GC.
Comparing genome copy yield for two media
We evaluated rAAV5 productivity in two different media ― HyCell™ TransFx-H and BalanCD™ HEK293 medium (Fujifilm Irvine Scientific). The titer was measured as GC/L (genome copy number per liter) and four replicates were used for the comparison.
We found a four-fold increase in GC/L for HyClone™ peak expression medium compared with BalanCD™ medium (p < 0.05) as shown in Figure 5. Yield of GC/cell was 2.6 × 104 GC/cell for BalanCD™ medium while for HyClone™ peak expression medium, we observed 6.8 × 104 GC/cell, which corresponds to more than a two-fold increase, data not shown.
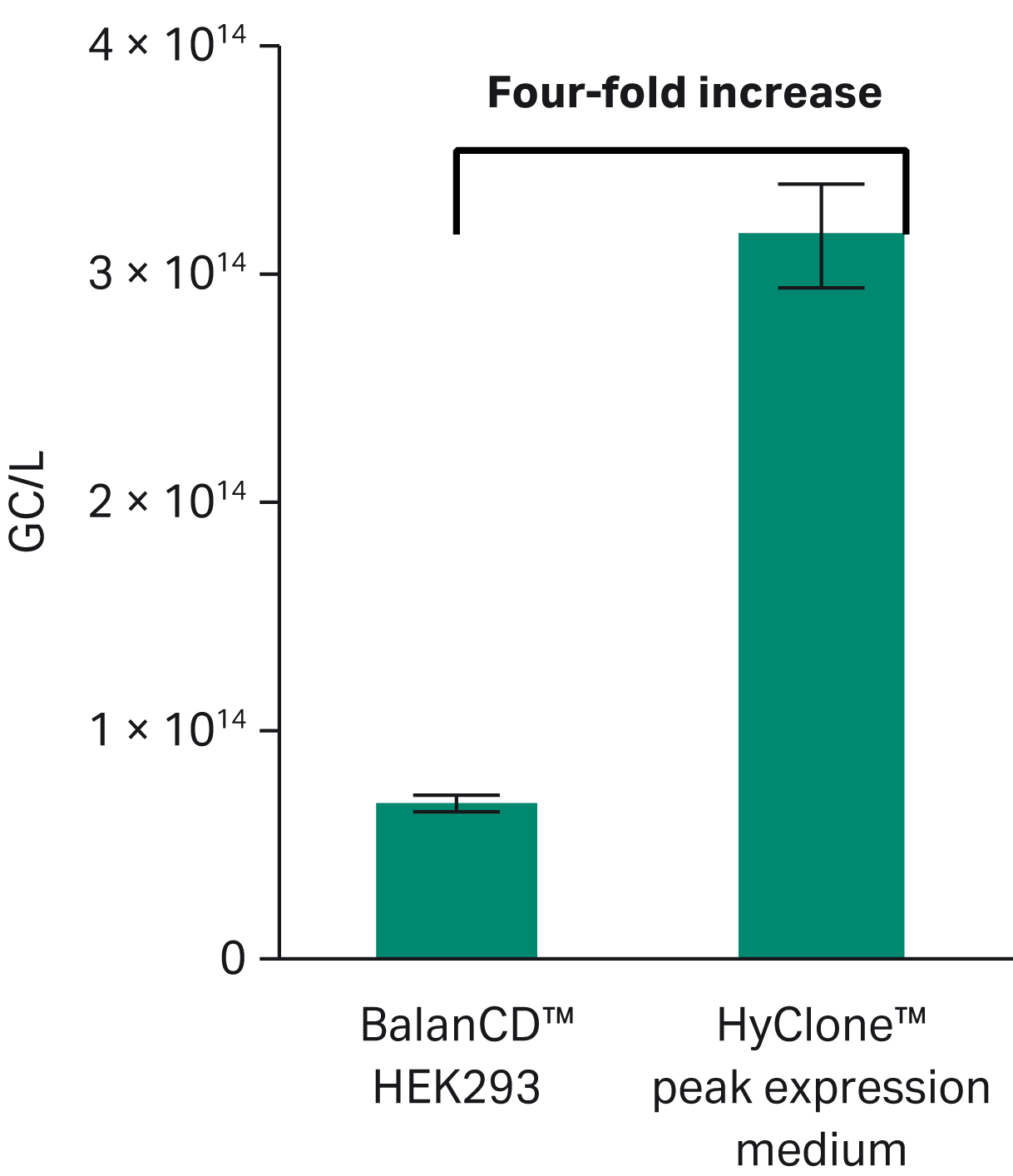
Fig 5. Comparison of genome copy yield for HyClone™ peak expression medium and BalanCD™ HEK 293 medium in rAAV5-GFP production (n = 4, p < 0.01). Statistical evaluation was performed using Student’s t-test.
Scaling up rAAV5 production in bioreactors
Finally, we scaled up productivity of rAAV5-GFP from the shake flasks to the 10 L scale using Xcellerex™ XDR-10 bioreactor followed by a further scale-up to 200 L using the XDR-200 bioreactor (Fig 6). The results show that the process is scalable between the 10 and 200 bioreactor scales with some yield loss from the shake flask cultures. The percentage GFP peaked at 48 h after transfection (2 d, data not shown).
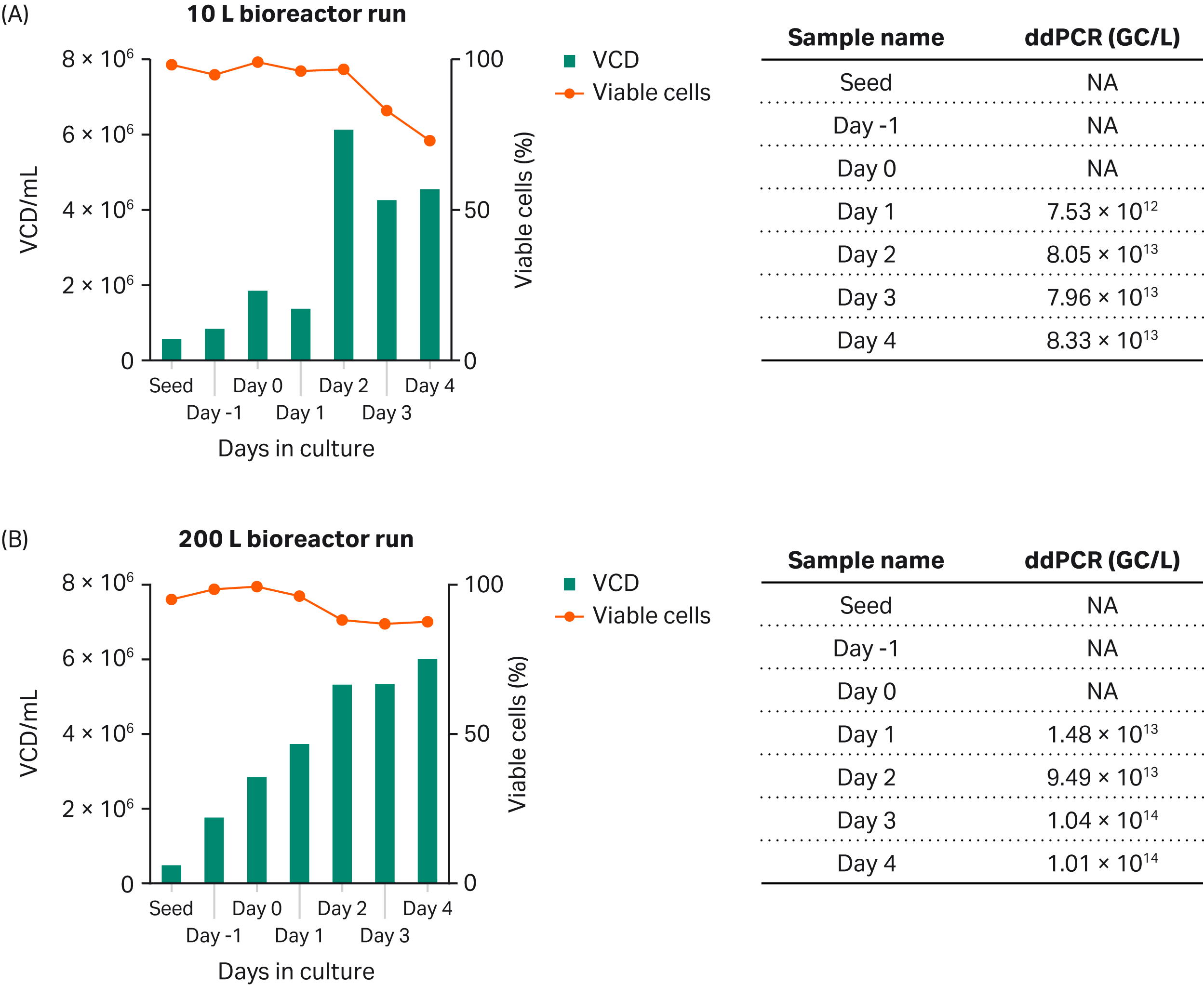
Fig 6. Production of rAAV5-GFP in 10 L culture using (A) XDR-10 bioreactor and 200 L culture using (B) XDR-200 bioreactor.
DNA-PEI complexing in complete culture media
To avoid aggregates, reduce shear stress, and promote cell growth in suspension cultures, polaxamers are frequently used in in growth media. We found that production of rAAV was below 1.8 × 1014 GC/L when complexing of PEI and DNA was done without Pluronic™ F-68 and above 1.8 × 1014 when Pluronic™ F-68 was present, although the difference in yield was not statistically significant (Fig 7).
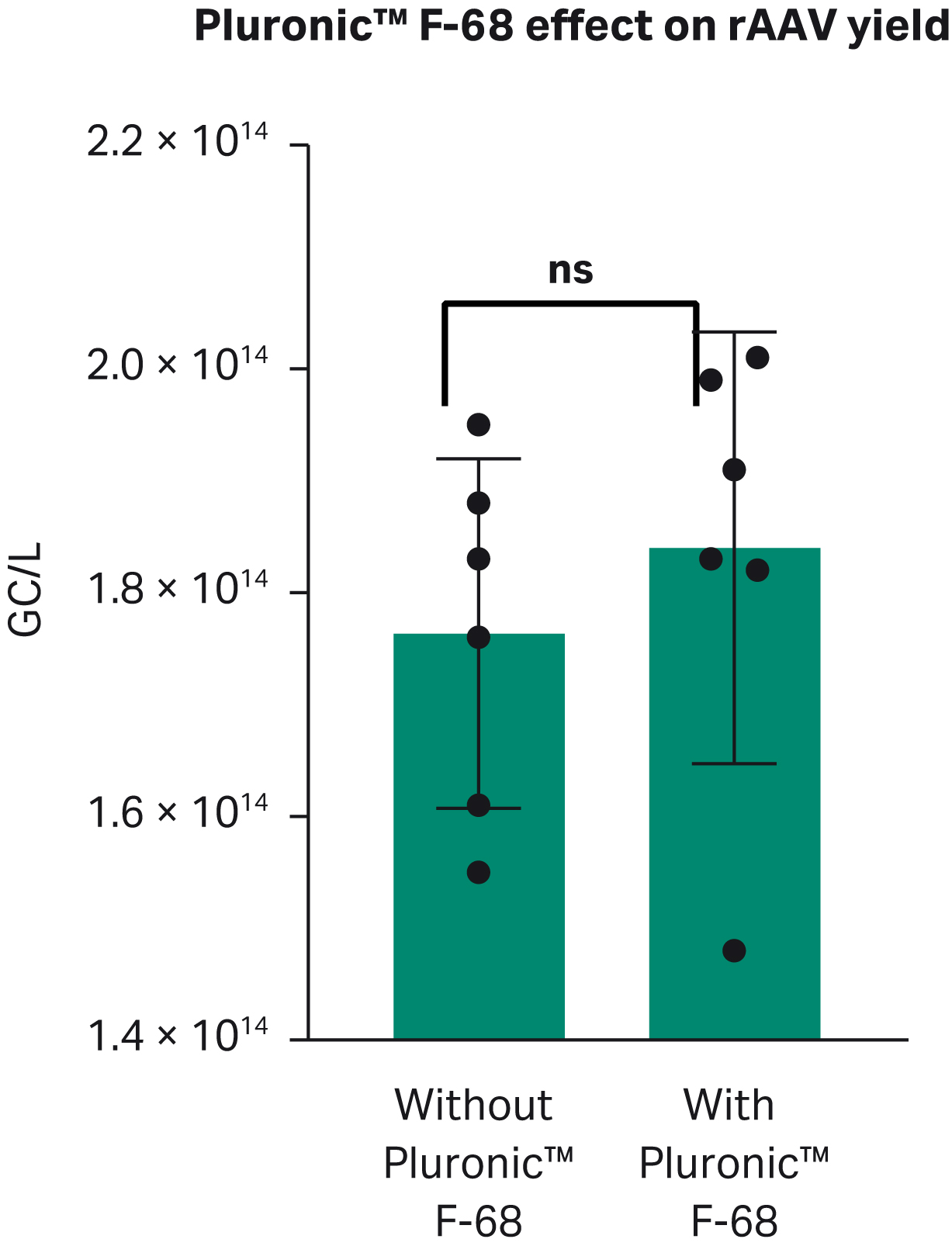
Fig 7. Effect of Pluronic™ F-68 on rAAV viral titer.
Conclusion
We observed the following about HyClone™ peak expression medium:
- The fully supplemented composition of the HyClone™ peak expression medium removes the need for additional culture materials and makes it more robust and reproducible in repeated experiments.
- Good recovery from cryopreservation and consistent culturing conditions.
- High-density culturing up to 9.0 × 106 of cells in shake flasks, which allows batch production of rAAV.
- Scale up from shake flask to 200 L is possible using the HyClone™ peak expression medium.
- We found a four-fold higher yield of rAAV productivity when compared to BalanCD™ HEK293 medium.
- Consistent DNA-PEI complexing in 10% of the production volume.
- Poloxamer had no effect on DNA-PEI complexing effectiveness.
Find out how you can develop and refine your AAV process for improved productivity.
Learn more about Cytiva’s solutions for upstream process development.
Learn more about HyClone™ peak expression medium.
References
(1) Mueller, C, Flotte, TR, Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15(11):858-863. doi:10.1038/gt.2008.68
(2) Flotte, T, Gene therapy progress and prospects: Recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2004;11:805-810. https://doi.org/10.1038/sj.gt.3302233
Acknowledgment
I would like to thank the Cytiva team for very helpful and constructive feedback while writing this application note. Special thanks go to Ann-Christin Magnusson, Vidya Murthy, and Camille-Germain Duhamel for their time and patience with this endeavor.
CY38428-24Jul23-AN