
Viral vector manufacturing shortage
Address the gap by understanding available strategies and how to select the most cost-effective solution.
Addressing the need for flexible manufacturing options aligned with your business strategy
Development of emerging and novel therapies is moving faster than ever. To keep pace among uncertainties about future manufacturing timing and scale, you need to weigh options. We know that with emerging therapies, one size doesn’t fit all. Boost your in-house expertise through collaboration to assess options that can offer flexibility in your process and manufacturing strategy.
Staying flexible in biomanufacturing can help you manage unknowns.
Build or buy | Manufacturing resources | Facility solutions
Whether you’re waiting for a production slot with a qualified CDMO or ready to build, a flexible strategy can help you scale quickly, to produce the right quantity at the right time. The decision to build, buy, or both requires a careful assessment of many factors. READ MORE
How to rapidly create single-use biomanufacturing capacity.
On-demand webinar: Outsourcing to accelerate your next milestone.

Address the gap by understanding available strategies and how to select the most cost-effective solution.

New market dynamics are reshaping how biologics are produced and sold. Here’s what six industry experts say about the way forward.

A flexible strategy can help you produce smaller scale, more potent molecules while supporting manufacturing of multiple products.

Choose a CDMO wisely to accelerate your programs while managing risk.
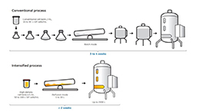
Explore available options to select a path based on molecule and facility fit needs.

Parallel operations afford the opportunity to develop a process in a parallel path using the resources of an external CDMO partner.
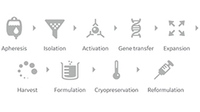
Services and manufacturing solutions for scalable and efficient end-to-end production.
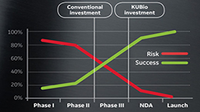
Meet changing market demands with flexible solutions to help manage process complexity.
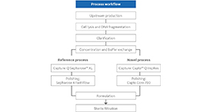
Rapidly add capacity and retain flexibility with modular end-to-end options.

Biologics contract development and manufacturing organization (CDMO).

From feasibility to biomanufacturing facility.