In marine chemistry research, scientists conduct water quality tests of oceans, lakes, rivers, and coastal water ways to track changes over time and understand the effects of human activity. These studies often involve filtration to separate fractions of a water sample that are indicative of the ecological state of a body of water.
Water quality testing? We’ve got you covered, find out more about our extensive range – download now!
Parameters to study water quality
Given the complexity of surface water composition both at the micro and the nanoscale, selecting the most relevant environmental parameters to study is key to retrieving the most useful information from collected samples.
At the molecular level, many researchers study the abundance of specific chemical elements in water samples. Carbon, nitrogen, and phosphorus content are fundamental indicators of the presence or potential for life in a body of water.
Dividing carbon content into different fractions enables a more precise description of the carbon present in a sample compared to total carbon. For example, inorganic carbon, found mostly in the form of carbonate, relates to atmospheric CO2 levels and ocean acidity. Organic carbon, such as that found in large biomolecules, is indicative of the amount of life present in the water.
At the microscale, there is a distinction between dissolved and particulate matter. When studying organic carbon, the dissolved and particulate fractions often have different origins. Particulate matter mostly consists of phytoplankton and organic detritus, whereas microorganisms tend to produce much of the dissolved matter.¹
The distinction made between fractions can be vital for certain types of studies. For example, dissolved carbon has a greater role than particulates in ocean acidification, making the former a key target for analysis in such studies.
Workflow for studying key parameters
There are a range of parameters relevant to testing water quality, the selection of which depends on both the aim of the study and sampling location (Table 1). Of these, dissolved organic matter (DOM) and particulate organic content (POC) are two of the main parameters that provide a measure of marine ecology.²
Table 1. Common parameters measured in surface water analysis| Acronym | Parameter |
TSS | Total suspended solids |
POC | Particulate organic carbon |
PON | Particulate organic nitrogen |
PIC | Particulate inorganic carbon |
DOM | Dissolved organic matter |
DIM | Dissolved inorganic matter |
DOC | Dissolved organic carbon |
DIC | Dissolved inorganic carbon |
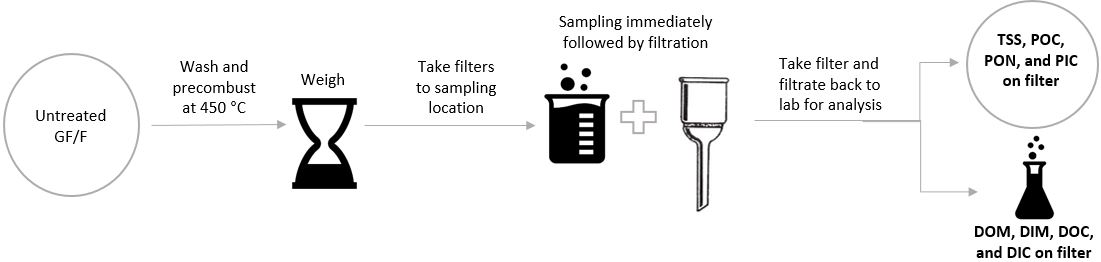
The precise workflow varies between the different parameters, but many involve the same preparative steps of treating a glass fiber filter before filtration (Fig 1). Filtration separates the dissolved and particulate fractions as filtrate and retentate, and enables analysis by the means appropriate to each.
Fig 1. Key steps of a workflow for determining dissolved and particulate matter in water samples.
In both POC and DOM measurements, glass fiber filters are ignited at 450° C and weighed before filtration. Ignition burns off any volatile substances and moisture that may be present and might otherwise influence downstream measurements.
In POC measurement, adding HCl to the filter after sample filtration removes dissolved carbonates. This treatment is followed by a drying step. A second weighing then allows for the calculation of retentate mass, leaving it ready for further elemental analysis, if needed.³
DOM can be analyzed from the filtrate by methods such as high-pressure liquid chromatography or mass spectrometry.⁴
Filter specifications
Glass fiber is a versatile inert material well suited for surface water filtration and sample collection due to several properties that could otherwise influence the result of an analysis. These properties include particle retention size, high loading capacity, fast flow rate, and stability at high temperatures.
A 0.7 µm retention size is common for water analysis and determines the minimum particle size retained. Uniformity of this retention size across multiple filters and batches of filters provides the consistency needed for samples taken over a period of time.
Small changes in filter mass can also influence measurements. The stability offered by glass fiber enables it to withstand ignition and minimize fiber loss during filtration. Fiber loss would otherwise make retentate mass measurements inaccurate and contaminate filtrate fractions.
The inert nature of glass fiber also minimizes the possibility of extractables influencing the analysis.
Manufactured from pure borosilicate glass, glass fiber filters, such as Whatman Grade GF/C and Whatman Grade GF/F, are well suited for use in surface water filtration and set a benchmark for particulate and dissolved matter analysis. The filters are available in a range of pore sizes and thicknesses and can retain particles down to 0.7 µm. Table 2 describes typical properties of GF/C and GF/F filters.
These glass fiber filters also have extremely low fiber loss and can withstand combustion up to 550 °C, providing the stability and consistency needed for accurate marine sample analysis.
To find out more about water filtration processes in marine chemistry or glass fiber materials in particulate and dissolved matter analysis, contact Cytiva Support or visit our Whatman Filter Selector to find the right filter for your needs.
Table 2. Typical properties of GF/C and GF/F| Property | GF/C | GF/F |
Nominal Air Flow Rate | 6.7 s/100 ml/in2 | 19 s/100 ml/in2 |
Nominal Thickness | 260 µm | 420 µm |
Nominal Basis Weight | 53 g/m2 | 75 g/m3 |
Maximum Recommended Temperature | 550 °C | 550 °C |
Material | Borosilicate glass | Borosilicate glass |
Binder Type | Binder-free | Binder-free |
Typical Particle Retention in Liquid | 1.2 µm | 0.7 µm |
Typical Water Flow Rate | 105 ml/min | 41 ml/min |
References
- Blondeau-Patissier, D. et al. Bio-Optical Properties of Two Neighboring Coastal Regions of Tropical Northern Australia: The Van Diemen Gulf and Darwin Harbour. Frontiers in Marine Science 4, 114 (2017).
- Alonso-González, IJ. et al. Lateral POC transport and consumption in surface and deep waters of the Canary Current region: A box model study. Global Biochemical Cycles 23, 1–12 (2009).
- Maciejewska, A. and Pempkowiak, J. DOC and POC in the water column of the southern Baltic. Part I. Evaluation of factors influencing sources, distribution and concentration dynamics of organic matter. Oceanologica 56, 523–548 (2014).
- Garrido Reyes, T. and Mendoza Crisosto, J. Characterization of Dissolved Organic Matter in River Water by Conventional Methods and Direct Sample Analysis-Time of Flight-Mass Spectrometry. Journal of Chemistry (2016).