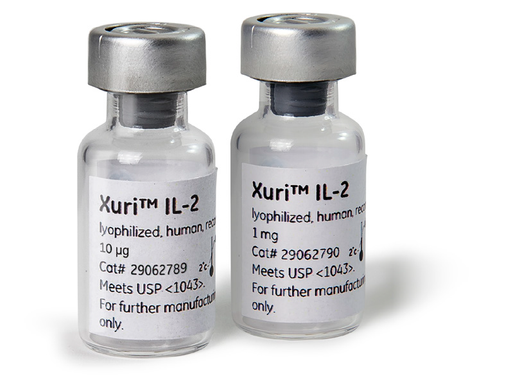
Xuri™ IL-2 growth factor
Interleukin-2 available in two formats – powder or liquid. This growth factor reliably activates T lymphocytes for efficient cell expansion. Meets USP <1043> for cell therapy ancillary material as applicable to supplier.


Xuri™ IL-2 growth factor
Interleukin-2 available in two formats – powder or liquid. This growth factor reliably activates T lymphocytes for efficient cell expansion. Meets USP <1043> for cell therapy ancillary material as applicable to supplier.
1. Pack size
2. Physical Form
Overview
Interleukin-2 available in two formats – powder or liquid. This growth factor reliably activates T lymphocytes for efficient cell expansion. Meets USP <1043> for cell therapy ancillary material as applicable to supplier.
- Gain peace of mind. Produced under a certified GMP license regularly qualified by licensing authorities. Sterility- and endotoxin-tested according to USP.
- Reproducible. No need to revalidate each new lot.
- Manage contamination risk. Easily connect Xuri IL-2 Syringe to a bag via Luer connection.
Functionality of this product is based on biological activity assays. Animal component-free. All Xuri reagents and media are compatible with Xuri Cell Expansion System.
Interleukin-2
Interleukin-2 promotes the growth, activity and maintenance of T and B lymphocytes. IL-2 is key in a number of cell and cell-based gene therapies.
Regulatory support
Regulatory support file (RSF) is available.
Quality statement
Xuri growth factors are manufactured, tested, and released in compliance with relevant cGMP guidelines. USP Chapter ‹1043› ‘Ancillary materials for cell, gene and tissue-engineered products’ has been followed in the design of these products.