This article is adapted from sponsored content originally published on https://www. cellculturedish.com
The COVID-19 pandemic brought mRNA vaccines to the spotlight with the rapid release of highly efficacious (94–95%) vaccines by Pfizer/BioNTech and Moderna. Once the sequence of SARS-CoV-2 was published, Moderna had its first candidates available in only 28 days (1). Full phase 1–3 trials and release of millions of doses were completed in months — much shorter than the years typically required for other vaccines (2).
Besides shortened development time and high efficacy (at least for COVID), there are other advantages for the use of mRNA for both prophylactic and therapeutic vaccines (3). One is the safety profile, which includes that the antigen is typically expressed for only a matter of days and can be modulated by the design of the mRNA. mRNA vaccines are also much more controllable than attenuated and vector vaccines. Unlike DNA-based approaches, mRNA vaccines do not require nuclear entry, so there is less risk of genomic integration and mutagenesis. Last, mRNA vaccines enable robust development of cellular and antibody responses, and these can be targeted to some degree by the design of the mRNA, the choice of delivery method, or other approaches.
There are also advantages in the production method, as synthesis is based on well-established in vitro transcription processes in a cell-free system (Fig 1). The cell-free system helps reduce cost, time, and manufacturing footprint. The plasmid DNA (pDNA) template for mRNA vaccines requires a cell-based fermentation step, but this is not a highly costly or time-consuming step. Moreover, mRNA offers flexibility, as vaccines for variants or multivalent vaccines can typically be manufactured without a significant change of production process.
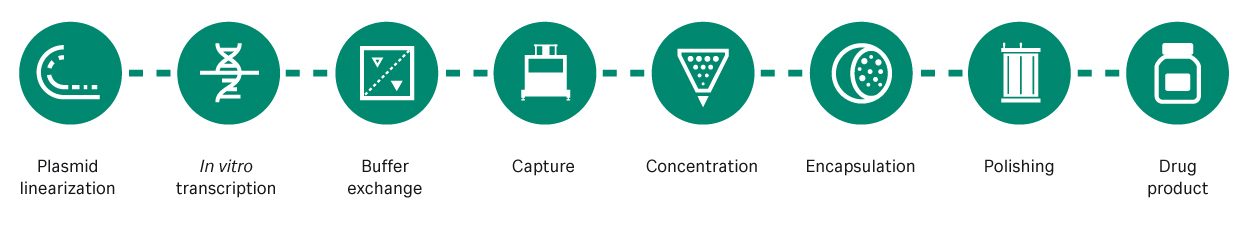
Fig 1. The mRNA manufacturing process. mRNA is transcribed from a linearized pDNA template then captured, concentrated, encapsulated, and purified for delivery to patients.
In other words, mRNA therapeutics is an exciting emerging therapeutic modality. But there are opportunities for improvement and maturation, especially in the areas of manufacturing, administration, and supply chain.
Due to its negative charge, mRNA does not easily enter cells, and it can be rapidly degraded by nucleases such as the enzyme RNase. Lipid nanoparticle (LNP) encapsulation, which is used in the current mRNA based COVID vaccines, helps mitigate this problem, as do other methods involving substitution of modified bases or design of the mRNA. Alternatively, physical methods such as electroporation can be employed. This approach has gained traction in ex vivo–administered therapeutic cancer vaccines but is not highly efficient.
Distribution of vaccine is another issue, as current mRNA vaccines require frozen storage. Alternative methods, such as lyophilization, are under study (4). Manufacturing also presents issues. One of the main challenges in mRNA processing is the lack of dedicated equipment and consumables fit for the relatively small volumes and large size of mRNA molecules, compared to traditional recombinant proteins. There is also room in technology development to improve scalability and process consistency.
Trends in RNA-based therapeutics
The potential of mRNA vaccines gained scientific attention in 1990 after the in vivo expression of a protein was observed after injection of naked mRNA into the skeletal muscle of a mouse (5). Since then, the industry has seen rapid development and expansion. Today, more than 140 clinical trials have looked at mRNA to address infectious disease, cancer, and a variety of other application areas.
Two forms of mRNA structure are currently being developed: conventional non-replicating mRNA and self-amplifying mRNA. Non-replicating mRNA vaccines have the conventional mRNA form, and do not have replication capability built into the mRNA sequence. The sequence of the antigen is flanked by untranslated (UTR) regions, a 3' poly(A) tail, and a 5' cap. The cap, UTR, open reading frame (ORF), and tail can be designed to up- or down-regulate expression, or to modulate immune response (4). Modified nucleotides such as pseudouridine and 5-methylcytidine can be used to lessen undesirable innate immune system responses and to increase translation efficiency (2,4). Thus, there are many aspects of the clinical response that can be modulated simply by the design of the mRNA.
Non-replicating mRNA vaccines are transient by nature and typically express antigen for a few hours or days (the cellular half-life of the Pfizer/BioNTech and Moderna vaccines is estimated to be 8-–10 hours). For some applications this can be beneficial, however for others such as systemic protein therapies, extended expression of a protein would be beneficial.
Self-amplifying mRNA (saRNA) approaches, which enable the mRNA to replicate, are under development. This, in turn, can extend the expression window to weeks (4–6). Typically, saRNA is based on the addition of viral replicase genes, in cis or trans configuration, from alphavirus, flavivirus, or picornavirus. These strategies can either increase expression level or lower mRNA dose requirements 10- to 100-fold. saRNA could potentially expand mRNA technology across many applications while lowering manufacturing demand. There are many areas of mRNA technology that are under development, and optimization and mRNA design are important aspects of current efforts.
Other RNA therapies outside of mRNA are being developed or have been approved. Among these are antisense oligonucleotides, which modify gene expression; small interfering RNA (siRNA), which also modifies gene expression via a different mechanism; aptamers, which can bind other ligands, including RNA; and guide RNAs, used for CRISPR targeting. Many of these RNA therapeutics share overlapping technology with mRNA vaccines. An example is Alnylam’s approved siRNA therapeutic Onpattro©, which uses LNP technology (2,3). Thus, RNA therapeutics overall is advancing rapidly in addition to mRNA vaccines.
Types of mRNA-based therapies
The COVID vaccines are prophylactic vaccines for infectious disease. Other prophylactic vaccines are in development against influenza, Zika, dengue, rabies, and Venezuelan equine encephalitis viruses, as well as bacterial infections such as Staphylococcus and tuberculosis (4,6). Unique approaches include expression of a neutralizing monoclonal antibody for chikungunya virus (4).
mRNA vaccines have also gained traction as a therapeutic approach for cancer. mRNA can elicit immune responses to mutated oncogenes or regulatory cancer genes such as TP53, which are shared across many cancers, in a therapeutic pan-cancer approach.
Other approaches for cancer include personalized therapy, where vaccines are developed for a person’s individual mutations. In this regard, a patient’s mutanome would be identified by next generation sequencing, and a handful of custom mRNA vaccines would be developed targeting the individual’s particular neoantigens (7).
Therapeutic cancer vaccines are advancing quickly in development, with over 70 clinical trials completed and more results expected in the next 2–3 years (5). Techniques under evaluation include the direct stimulation of antigen-presenting cells (APCs) via ex vivo electroporation of mRNA. Other approaches include direct intra-tumor injection, whole body approaches, and targeted organ approaches. Currently over 50% of clinical trials using mRNA focus on the treatment of melanomas and prostate and brain cancer (5). Thus, while numerous applications of mRNA vaccines are in various stages of development, targeting specific organs, tissues, and cells with LNPs is still under research.
Production and manufacturing bottlenecks
The quantities of mRNA needed for an application vary based on indication, potency of the approach, demand, and other factors. Customized, individualized applications may require the production of only milligram amounts of mRNA. Global needs may require much greater mRNA production capacity. For example, the current Pfizer/BioNTech and Moderna COVID vaccines contain 30 μg and 100 μg of non-replicating mRNA, respectively (1). For these vaccines, a production campaign of 1 billion doses would require the manufacture of 30–100 kg of highly purified cGMP mRNA, preferably via production batches of at least several grams each.
One of the most common bottlenecks in current manufacturing of mRNA is scaling. There is certainly a need for larger-scale production technology now that COVID products are reaching scales of billions of doses. We see a large interest in Cytiva’s FlexFactoryTM platform and KUBioTM box solutions, where a full start-to-finish solution can be tailored and delivered to customers. These solutions have been developed and delivered for mAb applications as well as for plasmids and viral vectors. However, there is also room for improvement in smaller scale cGMP manufacturing, as much of the current equipment is repurposed from the biotech industry and is designed for much larger scales than needed for mRNA. The industry could benefit from equipment specifically designed for mRNA cGMP manufacturing, including smaller scales.
Upstream manufacturing of mRNA is rather mature. cGMP-quality plasmid, polymerases, and enzymes needed for in vitro synthesis of mRNA are available but can be costly (5). Poly(A) tails can be created by inclusion in the template or by use of enzyme (4). Capping options include high-efficiency, co-synthesis reagents such as TriLink Biotechnology’s CleanCap© and enzyme treatment with high efficiency (4). However, even though mRNA vaccines have the potential to be less costly than other vaccine approaches due to the cell-free nature, currently they are more expensive to produce. To improve the overall cost profile, costs of GMP reagents, capping reagents, and propriety components such as LNPs need to be reduced.
The capacity constraints on the plasmids used as the starting template in the mRNA process are also a challenge, which this application area shares with the growing viral vector field. Companies are looking into ways to remove this bottleneck, and new technologies around cell-free processes for plasmid production could potentially improve this initial process step.
Downstream manufacturing, however, needs improvement. High purity of mRNA is required for efficient translation and to reduce undesirable immune responses (5). Impurities including enzymes, nucleotides, plasmid template, and aberrant RNA species necessitate multistep purification (5), and these processes are varied and in a state of development. Techniques such as precipitation, affinity oligo dT, ion pair chromatography (IPC) with or without cellulose, ion exchange chromatography, and tangential flow filtration (TFF) may be used (5). Thus, alternative purification ligands and refined purification approaches would greatly benefit the industry. Analytics could be improved as well.
While mRNA production is amenable to standardization and platforming, as has occurred in the monoclonal therapeutics industry, much current production is performed in multiple steps using fit-for-purpose equipment. Other aspects, such as single-use items and continuous processing strategies, would benefit this emerging industry.
The speed and potential cost gains of mRNA technology make it an interesting technology for personalized medicine, where vaccines are developed against a person’s individual cancer mutations. Many companies are working on integrated system mRNA processing solutions for this. Although many steps in the process are identical, challenges due to scale and cost remain. But development in this area continues and may reach the commercial stage in the future.
Finally, there is a need for greater understanding of the science of the process. For example, LNPs are typically formed in a rapid mixing process using microfluidic devices (2), which is more of an art than an established method. Greater understanding of the influences of the LNP ingredients and their effect on LNP stability, delivery, efficiency, immune response, and ultimately patient outcome would benefit the industry (2). The optimization of LNPs and other delivery technology is a critical attribute that can determine the ultimate success or failure of a therapeutic.
Encapsulation and delivery technologies
The use of nanostructures, such as LNPs, is common in mRNA therapeutics, as these generally deliver higher efficiency than naked mRNA and allow for a broad variety of administration routes. A challenge with nanostructure technology is that it is complex by nature, and it involves many potential ingredients with many possible clinical outcomes. There is incomplete understanding in this regard. Nanostructure properties are critically important to clinical outcomes and include protection of nucleic acids, controlled release of RNA inside the cell, cell and tissue selectivity, translation efficiency, toxicity, and long term stability (2).
Nanostructures are sophisticated and may be composed of several components, such as common lipids, polymers, proteins, cholesterol, or custom proprietary components such as ionizable lipids (3,4). Often, conjugates such as polyethylene glycol–lipids are used. Each of these components influences the structural properties. For example, polymer content can control particle size and affect efficiency and cell tropism. Structural lipids, such as cholesterol, can affect particle stability. Empty nanoparticles without payload can form if not mixed correctly. Thus, nanostructure composition and formation are critical for desired clinical effect (2). Currently, LNPs are the leading non-viral delivery system for many systems, including gene therapy (2).
There are other delivery methods under study and development. Exosomes are thought to use a receptor, and may offer more efficient uptake, greater specificity, and fewer side effects (8). This a promising early area of research. Other areas include conjugated RNA, such as GalNac-siRNA, which has been shown to target liver hepatocytes (9). Likewise, GALA-peptide conjugated mRNA has been shown to improve uptake in APCs (10). Other approaches are under evaluation to increase target specificity or to improve cellular uptake.
Naked mRNA has been evaluated for cancer therapy by approaches including direct injection to the tumor. Generally, naked RNA is considered less efficient than other methods, but it is easy to prepare, as it requires only a buffer (4,11). In some applications, the intrinsic high immunogenicity of naked mRNA may provide benefit via boosted adjuvant activity (11).
mRNA industry perspectives
With the arrival of the COVID-19 pandemic, mRNA for prophylactic vaccines took the public spotlight due to urgent need. These vaccines demonstrated the promise of mRNA therapeutics through their quick developmental timeline and high efficacy.
While COVID vaccines are notable, most mRNA vaccines investigated to date have been focused on cancer therapy, and dozens of clinical trials have been completed or are ongoing. Many of the ongoing trials are for personalized therapeutic cancer vaccines and should be completed in the next 2–4 years. Promising results in this area could further advance the mRNA industry.
Moreover, many therapeutics are in early development, across diverse areas that will have high impact if successful. Success in mRNA therapies could potentially displace less effective therapeutic approaches, such as vaccines for influenza, tuberculosis, or other applications.
Summary
mRNA therapy is a rapidly growing field, with too many applications in development to detail in this brief report. The technology offers great benefit and potential for infectious diseases and personalized medicines due to its advantages in flexibility, cost, and speed of development. There are still challenges to overcome to fully realize the potential of this technology, but the COVID-19 pandemic publicly demonstrated the promise of mRNA therapies. The future looks bright for this emerging industry.
- Teo SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2021(Apr 12);8971900211009650. doi:10.1177/08971900211009650.
- Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021 (Mar);170:83-112. doi: 10.1016/j.addr.2020.12.014.
- Schoenmaker L et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021 (May 15);601:120586. doi: 10.1016/j.ijpharm.2021.120586.
- Xu S, Yang K, Li R, Zhang L. mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020 (Sep 9);21(18):6582. doi: 10.3390/ijms21186582.
- Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021 (Apr 15);39(16):2190-2200. doi: 10.1016/j.vaccine.2021.03.038.
- Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019 (Apr 10);27(4):757-772. doi: 10.1016/j.ymthe.2019.01.020.
- Vormehr M, Schrörs B, Boegel S, Löwer M, Türeci Ö, Sahin U. Mutanome engineered RNA immunotherapy: towards patient-centered tumor vaccination. J Immunol Res. 2015(2015);595363.doi: 10.1155/2015/595363.
- Tsai S-J et al. Exosome-mediated mRNA delivery for SARS-CoV-2 vaccination. Preprint at bioRxiv 2020 (Nov 6). doi: 10.1101/2020.11.06.371419.
- Springer AD, Dowdy SF. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018 Jun;28(3):109-118. doi: 10.1089/nat.2018.0736.
- Lou B, De Koker S, Lau CYJ, Hennink WE, Mastrobattista E. mRNA polyplexes with post-conjugated GALA peptides efficiently target, transfect, and activate antigen presenting cells. Bioconjug Chem. 2019 Feb 20;30(2):461-475. doi:10.1021/acs.bioconjchem.8b00524.
- Zeng C, Zhang C, Walker PG, Dong Y. Formulation and delivery technologies for mRNA vaccines. Curr Top Microbiol Immunol. 2020 (Jun 2);10.1007/82_2020_217. doi: 10.1007/82_2020_217.