Article inspired by a Tapas and TECH Talks digital event. Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) team members shared peer-to-peer insights.
Cell lines have transformed scientific research. Also, they are used to increase titer and allow process developers to move molecules into the clinic faster. Bioprocessing often uses well-characterized cell lines to achieve processes that are both robust and reproducible. Optimizing the cell line can speed up process characterization and validation activities for scale-up by predicting manufacturing behavior at an early stage.
In a CAR T workflow, patient material is enriched for T cells and expanded in bioreactors to get the appropriate dosage. The chimeric antigen receptor (CAR) is introduced to the cells through lentiviral vectors (LV). However, these therapies are expensive, and LV production represents a large portion of these costs. High manufacturing costs for LV are driven by low efficiencies from limited scalability in adherent cell culture, as well as requirements for large manufacturing spaces and several manual manipulations that increase labor costs. At the Centre for Advanced Therapeutic Cell Technologies (CATCT) scientists are working to address obstacles like these that hinder commercialization of cell therapies. One project is aimed at reducing LV production costs.
Points to consider for lentiviral vector production
Before producing LV, it is important to carefully consider which system you will use: transient or stable transfection. This decision will inform the type of cell line you can use to produce the LV.
Transfection method
There are two main strategies for producing LV in mammalian cells: transient transfection and stable transfection. Transient transfection introduces the nucleic acid for a limited time and does not integrate into the genome. Although the nucleic acid does not get passed on, transient methods have the advantage that high copy numbers can be transfected, which typically results in high levels of expression within four days. In contrast, stable transfection introduces DNA that persists through the progeny since the DNA is incorporated into the genome. However, the cells need to undergo a development time for selection and isolating appropriate clones.
Despite the longer timeline, stable transfection is more amenable to producing a good manufacturing practices (GMP)-grade cell line. This is true because it allows the cells and the process to be well characterized. Further, the use of inducible promoters can eliminate the need for GMP-grade transfection reagents. Choosing stable transfection means that you can convert a ‘host cell’ into a ‘packaging cell’ or a ‘producer cell.’
Type of cell line
One strategy for simplifying, and thus reducing cost, during lentivirus production is to convert the lentivirus-producing cell line from the ‘host cell' or chosen mammalian cell line, which require transient transfection of multiple plasmids, into a ‘packaging’ cell. This is accomplished by genetically modifying it through stable transfection to express the LV Gag-Pol and Rev genes, and typically the VSV-G LV capsid gene as well. Most of the viral genetic material is stably expressed by the packaging cell, so lentivirus can be produced using a single plasmid transfection of the gene of interest. This allows flexibility to choose the gene of interest to incorporate into the lentivirus in order to facilitate various applications.
For manufacturing, it would be ideal to convert the packaging cells to ‘producer cells’ by further modifying their genomes to also include the therapeutic transgene with inducible promoters to signal for LV production. Producer cells allow LV to be produced without the need for additional transfection reagents.
Here is the general workflow and a case study for cell line development.
Stages in cell line development
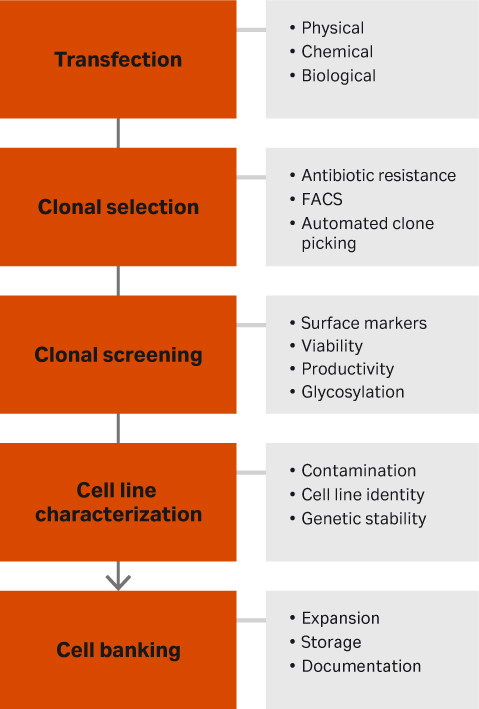
A typical workflow follows five stages (Fig 1). Here we explain the purpose of each one and offer up some tips.
Fig 1. Overview of a standard workflow for developing a cell line to produce a producer cell line for lentiviral vector.
- Transfection. This step gets your gene of interest inside the cells. It can be done through physical means (e.g., electroporation, nano needling, or cell squeezing), chemical methods (e.g., liposomes or cationic polymers), or biological (viral vectors like lentivirus or retrovirus).
- Clonal selection. After the gene of interest has been inserted into the cell, clones must be selected to isolate the cells that contain that gene. The simplest method is through antibiotic resistance. If there are surface markers, fluorescence activated cell sorting (FACS) may be an option as well as colony pickers. The latter pick individual clones from your cell suspension and place them into individual wells.
- Clonal screening. After selecting for the cells that contain the gene of interest, the cells will be screened for high producers. Depending on the desired output of the cells, options include observing surface marker expression, cell viability, productivity, and glycosylation.
- Characterization. High-producing cell clones must be checked for contamination through mycoplasma testing, sterility, and virus testing. The cells also must be characterized by DNA barcoding, fingerprinting, and karyotyping. It is also important to ensure genetic stability through DNA sequencing looking for short tandem repeats as well as gene copy numbers.
- Cell banking. The final step is creating the cell bank. Cells must first be expanded by selecting appropriate selection media and optimizing cell culturing methods. When creating the cell bank, it is important to have all the necessary documentation to make regulatory submission paperwork easy, especially for GMP manufacturing.
Case study highlights
Goal: Develop a stable packaging cell line that could be widely adopted by any sort of LV-based gene therapy.
The first step in the overall experimental plan is to identify any genetic roadblocks that are suppressing high LV titers. A genetic screen will be used to determine high LV producers. Once these roadblocks are identified, the packaging cell line will be engineered to remove these roadblocks. The final step will be a proof-of-concept step to generate a producer cell line from this engineering packaging cell line.
Typical formats for genetic screening are pooled and arrayed. In a pooled screen, an entire library can be applied to the cells, which allows a high throughput. After a bulk selection for transfected cells, the relevant constructs can be sequenced to identify genes of interest. On the other hand, an arrayed format is more methodical. It is more laborious than a pooled screen, with the ability to perform a well-by-well screen with single variants per well. The arrayed format may be more amenable to complex phenotypes, but the size of the screen is limited due to the nature of a well-by-well screen.
To develop a cell line for lentiviral production, a CRISPR-based pooled screen was initially employed. However, no lentivirus was produced using this approach, due to the complexity of designing a plasmid that contained a transgene for use both as a guide RNA (sgRNA) and as a plasmid to be repackaged in the lentivirus. Instead of redesigning the plasmid, an arrayed genetic screen format was investigated as an alternative option.
Both a CRISPR-based and an siRNA-based screen were considered for the arrayed screen. Unfortunately, the CRISPR-based approach did not yield the desired knockout efficiencies, but the siRNA-based approach yielded high knockdown efficiency. However, one of the limitations of an siRNA-based screen is that it only knocks down expression, which may not translate directly to knocking out the gene. With this risk, it would be ideal to work on optimizing the CRISPR-based approach, but it was informative to observe the efficacy of the siRNA-based screen.
As mentioned, an arrayed screen cannot apply an entire library. Hence, in order to identify targets for the arrayed screen, an RNA-Seq analysis was performed on two cell lines producing lentivirus. The RNA-Seq analysis identified hundreds of commonly upregulated genes between the two cell lines during lentivirus production. These genes were identified as the target for the arrayed screen.
View the webinar for more details on this robust approach to cell line development.
About Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT)
This work was performed at the Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) in Toronto, Canada. This center was conceived in January, 2016 as a joint collaboration between Cytiva, CCRM, and the government of Canada. The center was tasked with developing the next generation of solutions for effective manufacturing of cell and gene therapy products to make these treatments more accessible to patients. The center includes associates with expertise in process development and optimization, manufacturing, and many other specialties. Learn more about Fast Trak services for cell and gene therapy.