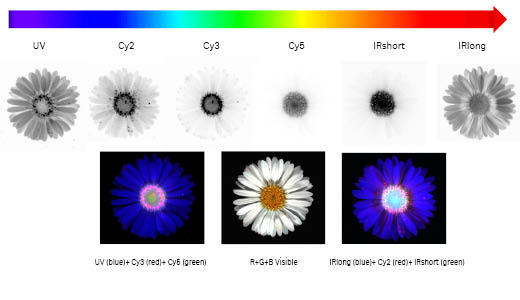
| Applications |
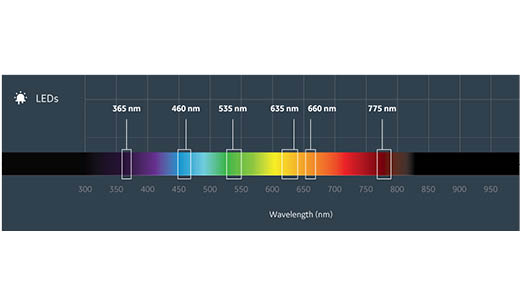
Light sources |
ImageQuant 800 |
ImageQuant 800 UV |
ImageQuant 800 OD |
ImageQuant 800 Fluor |
| Chemiluminescence with color marker overlay |
Epi-white |
√ |
√ |
√ |
√ |
| Gel documentation |
Epi-white |
√ |
√ |
√ |
√ |
| Stained gels |
Epi-UV |
|
√ |
√ |
√ |
| Optical density (OD) measurements |
Trans-white |
|
|
√ |
√ |
| RGB fluorescence imaging of blots and gels |
Epi-RGB |
|
|
|
√ |
| IR short and IR long fluorescence imaging of blots |
Epi-IR short, Epi-IR long |
|
|
|
√ |
| GxP software extension for regulated environments (optional) |
|
√ |
√ |
√ |
√ |
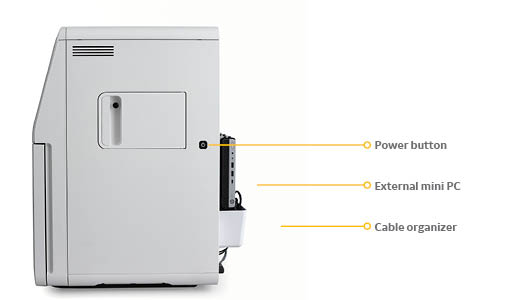
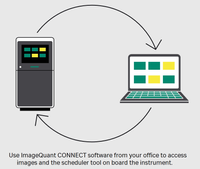
ImageQuant 800 systems are equipped with a large, bright 12.1-inch touchscreen, dark sample cabinet with two tray positions, optical density (OD), cooled CCD-based camera system, filter wheel, and light-emitting diode (LED) light sources. The system is controlled using a mini external computer which neatly fits at the back of the system (Fig 2), reducing footprint. A side door allows users to add their own customized fluorescent filters. GxP module available for regulatory labs.
View Data file | Explore the system in 3D
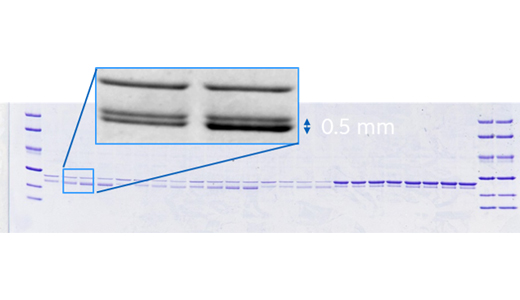
Reveal more with SNOW imaging mode—up to 2× greater sensitivity
Detect faint bands with precision using SNOW (signal-to-noise optimization watch), an automated imaging mode that delivers up to 2× higher sensitivity—without sacrificing resolution or image quality.
This intelligent imaging mode continuously updates the image in real time, allowing users to monitor progress as it happens. SNOW automatically stops when the optimal signal-to-noise ratio is reached, ensuring the best possible image with minimal hands-on time.
- Enhanced sensitivity: Detect up to twice as many weak bands without sacrificing resolution.
- Preserve image integrity: Avoid saturation while capturing both strong and faint signals.
- Wider dynamic range: Extend linearity by preventing overexposure.
- Hands-free optimization: Auto-stop feature eliminates the need for manual exposure adjustments.
- Streamlined workflow: No more trial-and-error—just clear, high-quality results.
Image integrity checker
To support researchers in maintaining the highest standards of transparency and reproducibility, Cytiva offers the Image Integrity Checker—a free, easy-to-use tool that helps validate Western blot images before publication. Read more and download the software here.
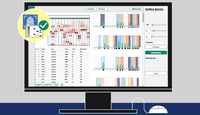
ImageQuant TL 11 analysis Software
ImageQuant™ TL is an intuitive and powerful software for quantitative image analysis in gel and blot imaging, multiwell plate analysis, and colony counting. It supports workflows for protein research and is compatible with Amersham™ and Typhoon™ imaging systems.
- Gel & Blot Analysis: Auto lane/band detection, background subtraction, normalization.
- Normalization Options: Total protein, housekeeping protein, in-channel, or single band.
- Lane Comparison: Compare samples, assess purity, detect artifacts.
- Additional Tools: Array analysis, colony counting, general image tools.
- Advanced Imaging: Pixel-level zoom, export-ready images.
- Compliance Support: GxP module, audit trails, secure login.
- System Integration: Link chromatograms from ÄKTA™ to gel lanes.
View the ImageQuant™ TL software page
ImageQuant™ GxP Software – Designed for Compliance, Built for Confidence
To support laboratories operating under stringent regulatory frameworks such as FDA 21 CFR Part 11 and EU GMP Annex 11, Cytiva offers ImageQuant GxP Software—a robust solution that ensures data traceability, accountability, and integrity throughout your imaging workflow.
- Secure user access: Windows®-based login and user group management for controlled system access.
- Comprehensive audit trails: Electronic records and event logs with time-stamped entries for full traceability.
- Data integrity assurance: Seamless integration with ImageQuant TL analysis software via digital handshake to ensure image date integrity.
- End-to-end traceability: Link imaging data to pre-imaging steps using lab notebook reference number fields.
- Regulatory-ready: Includes full documentation, validation support, and independent third-party certification for 21 CFR Part 11 compliance.