Chronicle™ automation software
Chronicle™ automation software provides a unified digital platform to monitor facility manufacturing operations and supply chain logistics





Chronicle™ automation software
Chronicle™ automation software provides a unified digital platform to monitor facility manufacturing operations and supply chain logistics
Renewal
Overview
Chronicle™ automation software connects Cytiva and third-party instruments. From process development through commercial GMP manufacturing, pre-GMP and GMP plans have been designed to support you.
Digitize records to reinforce GMP compliance and improve sample security with Chronicle™ automation software. eSOPs manage deviations, promote adherence, and provide training. Chronicle™ automation software has been independently audited against GAMP5, 21 CFR Part 11 and EU Annex 11. Plus, Chronicle™ automation software can support two different environments—the cell therapy and the biologics environment—to better fit your specific needs.
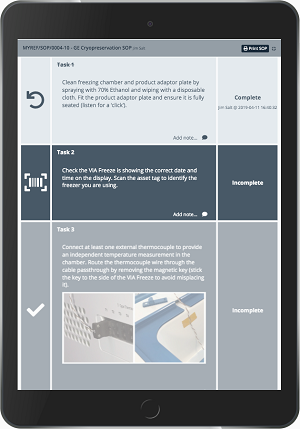
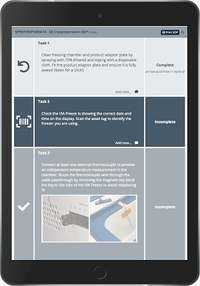
- Implement electronic batch records that comply with regulatory requirements, reviewing deviations efficiently with automated workflows to support release by exception.
- Optimize operators’ time and help reduce mistakes with electronic standard operating procedures (eSOPs).
- Schedule procedures by reserving instruments and checking availability on a built-in calendar.
- Trace all your products and consumables with stock control, barcode label printing, and scanning. Obtain a Certificate of Analysis (COA) automatically for many Cytiva cell therapy reagents and plastics.
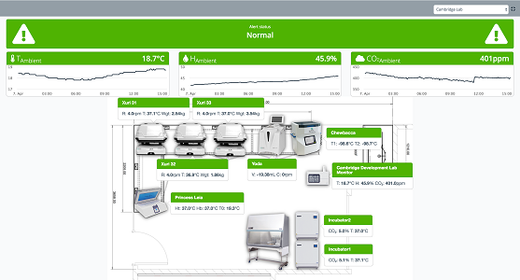
- Connect third-party instruments to upload and visualize instruments data.
Chronicle™ automation software provides a unified digital platform to monitor your facility manufacturing operations and supply chain logistics. Plus, electronic batch records will trace every manufacturing step.
Ready to increase productivity with automation? Select a plan that matches your stage of development: pre-GMP and GMP software packages allow you to adopt digital solutions as early as process development and can scale with you throughout commercialization. Click “read more” for details on the plans, or contact us to request a demo or ask specific questions.
Choose the plan that meets your needs
| Pre-GMP | GMP | |
|---|---|---|
| Recommended use | Manufacturing automation and the ease of tech transfer for a pre-GMP environment | Optimized for GMP-manufacturing at scale |
| Hosting | Via secure cloud—shared with other pre-GMP users | Via secure cloud—private, dedicated to one customer |
| Software updates | Automatically applied when available | Only applied after customer’s approval |
| Includes | Virtual Chronicle™ automation software gateway | •Virtual Chronicle™ automation software gateway •Validation support package •Access to a account (same version as the GMP account) •Access to a pre-GMP account (with unlimited users, for as long as the GMP subscription is active) •Standard service-level agreement (SLA) |
| Pricing | •Pre-GMP base annual subscription •Optional annual subscription for 5,10, or unlimited number of additional users |
• One-time hosting setup fee • GMP base annual subscription • Optional annual subscription for 10, 25, or unlimited number of additional users |