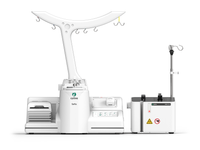
Sefia™ expansion system
Automated and functionally closed cell therapy manufacturing solution covering cell activation, transduction and expansion.





Sefia™ expansion system
Automated and functionally closed cell therapy manufacturing solution covering cell activation, transduction and expansion.
Overview
Sefia™ expansion system combines three hardware components, dedicated application software and dedicated single-use kits to provide an automated and functionally closed solution to standardize and help secure your cell therapy manufacturing process.
- Streamlined manufacturing. Using the same system via a single procedure to cover three consecutive steps of your manufacturing workflow.
- Flexibility to meet your process needs. Customizable parameters and specific kits enable the system to adapt to your individual process and accommodate your chosen reagents and media.
- Designed to increase productivity. Reduce operator touchpoints and labor hours with high level of automation over three consecutive steps to optimize your resources and costs.
- Features designed to reduce batch failure risks. Functionally closed single-use kit keeps contaminants out. Control your process with real-time lab dashboards and alert notifications.
- Easy integration. User-friendly design of applications and consumables. Centralized data traceability, consistent setup duplication and regulatory compliance thanks to Chronicle™ software connectivity.
Sefia™ expansion kit is available in two types (FEP: fluorinated ethylene propylene and Si: silicone) to meet different customer needs.
Sefia™ expansion system Universal application offers high flexibility with customizable parameters to enable the user to fit the procedure to their specific needs. Universal application covers three consecutive steps of your cell therapy manufacturing: cell activation, transduction and expansion. You can start a procedure with Universal application with cellular product using a mass of between 5 and 250 g. Universal application default parameters are designed to execute one of many possible types of workflow, typically taking between 5 to 12 days. The cells are cultured in static culture vessels with temperature and CO2 concentration control and monitoring. Sefia™ expansion system can be used with your current reagents and media. It has been tested with market leading activators and media as well as various types of viral vectors (lentivirus and retrovirus). Different methods can be used to support the cell expansion: batch, fed-batch or perfusion. At the end of the procedure, the genetically modified and expanded cells will be harvested in a bag up to 1200 g.
The Sefia™ expansion system can be connected to Chronicle™ automation software to monitor cell therapy manufacturing operation and supply chain logistics. Chronicle™ software enables the digitization of manufacturing documentation by generating electronic batch manufacturing records (eBMRs) through the use of electronic standard operating procedures (eSOPS). It also offers the ability to monitor instrument data in real-time and receive alert notifications via SMS and emails. Chronicle™ software centralizes the creation, approval, and deployment of parameter groups on connected Sefia™ expansion systems. Chronicle™ software supports cell therapy process development and manufacturing in compliance with FDA 21 CFR Part 11 and EU Annex 11.