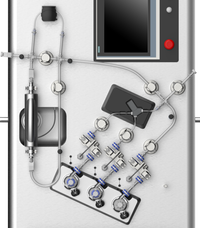
NanoAssemblr™ Blaze cartridges and tubing kits
Single-use cartridges with NxGen™ technology and tubing kits for lipid nanoparticle (LNPs) process development
NanoAssemblr™ Blaze cartridges and tubing kits
Single-use cartridges with NxGen™ technology and tubing kits for lipid nanoparticle (LNPs) process development
1. Dilution
2. Product type
Overview
NanoAssemblr™ Blaze cartridges and tubing kits are validated for single-use, reducing the risk of cross-contamination during process development of LNP formulations. Starting June 2025, customers can purchase the new NxGen™ Blaze cartridges for the NanoAssemblr™ Blaze nanoparticle formulation system and will have the option to reuse them.
- Automate In-line dilution: Cartridges are available with in-line dilution, simplifying LNP workflows and saving time while ensuring particle stability.
- Reproducible scale-up: NxGen™ technology ensures efficient transfer of critical process parameters (CPP) during scale-up from preclinical development to clinical and commercial production.
- Flexible flow rate options: NxGen™ 400 and NxGen™ 500 cartridges support flow rates from 4 – 115 mL/min using the same NxGen™ mixing architecture but in different dimensions, enabling flexibility for formulation-specific process parameters.
- Closed-system environment The single-use tubing kit enables connection to external vessels and supports a closed-system environment on the NanoAssemblr™ Blaze+ system, allowing optimization of manufacturing processes used during clinical development and minimizing risk.
saRNA-LNPs are biologically potent in vitro and in vivo, inducing expression of SARS-CoV-2 antigen and robust immune responses. A) BHK 570 cells were transfected with decreasing amounts of saRNA-LNPs and B) the percentage of cells expressing SARS-CoV-2 spike protein was measured using an anti-spike conjugated AlexaFluor488 antibody with 95% confidence intervals in shaded areas. C) EC50 values were similar across systems. Error bars represent 95% confidence intervals. D) BALB/c mice were used for a 42-day prime and boost dose study. E) Robust SARS-CoV-2-specific IgG responses in serum were observed at day 21 and 42 post-injection for each condition. Error bars are 1 standard deviation. 1x PBS vs instrument comparison p-value for a given time point using post-hoc Tukey test after one-way ANOVA (p≤.05: *, p≤.01: **, p≤.001: ***, p≤.0001: ****).