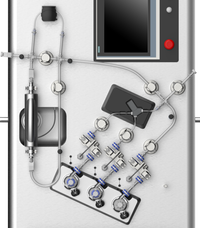
NanoAssemblr™ GMP System for nanoparticle formulation
Ready-to-use formulation system with a single-use fluid path for the clinical development of lipid nanoparticles (LNPs)
NanoAssemblr™ GMP System for nanoparticle formulation
Ready-to-use formulation system with a single-use fluid path for the clinical development of lipid nanoparticles (LNPs)
Overview
- Single-use fluid path: Reduces the risk of cross-contamination during the GMP manufacture of RNA-encapsulated LNPs.
- In-line dilution: Automated, in-line dilution of LNPs simplifies workflows and ensures particle stability.
- Reproducible: Scalable NxGen™ technology provides precise control of mixing parameters, resulting in consistent flow rates for robust particle formation.
- Complete documentation and traceability: Standard operating procedures (SOPs), qualification protocols, and material traceability reports enable technology transfer and facilitate compliance.
Have you seen Cytiva’s newest system for clinical and commercial production of LNPs? Meet the NanoAssemblr™ commercial formulation system.
NanoAssemblr™ GMP System overview
Learn how the NanoAssemblr™ GMP System enables the clinical development of LNPs.
Single-use fluid path
The NanoAssemblr™ GMP fluid path includes the NxGen™ cartridge, connective tubing, and pump head consumables and accessories. It comes with a traceability and material report to support regulatory and quality audits for GMP manufacturing of genomic medicines.
Scalable NxGen™ technology
NxGen™ technology enables controlled and reproducible mixing conditions through its innovative mixing architecture, consisting of unique toroidal structures within the flow route, and is designed to enable the transfer of critical process parameters (CPPs) during scale-up using the NanoAssemblr™ family of formulation systems.
Enable consistent critical quality attributes (CQAs) from preclinical to clinical development
Self-amplifying RNA (saRNA)-LNPs prepared using NxGen™ technology maintain physicochemical characteristics throughout scale-up to clinical development. Size, polydispersity index (PDI), and encapsulation efficiency are consistent using A) the NxGen™, NxGen™ 500, and NxGen™ commercial cartridge 48 L/h and B) from Ignite+™ for early preclinical development through to the GMP System and the commercial formulation system for clinical development. Error bars represent 1 standard deviation and comparison values are from a post-hoc Tukey test after one-way ANOVA.
Minimize process development by the direct transfer of CPPs
saRNA-LNPs are biologically potent in vitro and in vivo, inducing expression of SARS-CoV-2 antigen and robust immune responses. A) BHK 570 cells were transfected with decreasing amounts of saRNA-LNPs and B) the percentage of cells expressing SARS-CoV-2 spike protein was measured using an anti-spike conjugated AlexaFluor488 antibody with 95% confidence intervals in shaded areas. C) EC50 values were similar across systems. Error bars represent 95% confidence intervals. D) BALB/c mice were used for a 42-day prime and boost dose study. E) Robust SARS-CoV-2-specific IgG responses in serum were observed at day 21 and 42 post-injection for each condition. Error bars are 1 standard deviation. 1x PBS vs instrument comparison p-value for a given time point using post-hoc Tukey test after one-way ANOVA (p≤.05: *, p≤.01: **, p≤.001: ***, p≤.0001: ****).