Allegro™ single-use final filling assemblies
Customized final filling assemblies for aseptic filling of drug products




Allegro™ single-use final filling assemblies
Customized final filling assemblies for aseptic filling of drug products
Overview
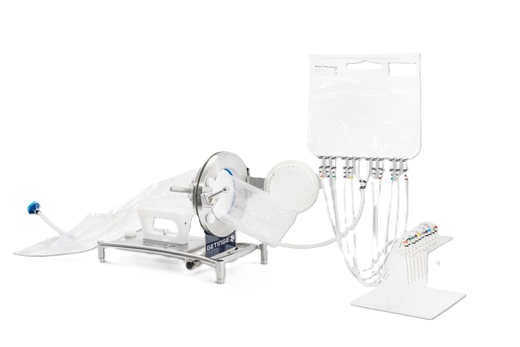
The Allegro™ single-use final filling assemblies are engineered to minimize the risk of contamination, thereby enhancing patient safety.
- Customized solutions to meet your process needs
- Use of Advanced Central Management System (ACMS) for fast and flexible design in adherence to Quality by Design (QbD) principles
- Assembly design of system to optimize filling line productivity, flexibility, and process robustness
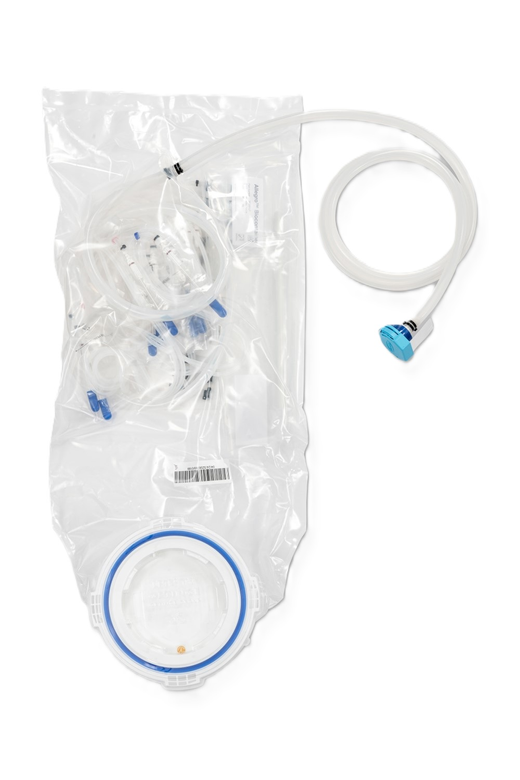
We tailor each filling assembly to meet specific accuracy requirements while ensuring ease of use for operators, even in constrained environments such as isolators or restricted access barrier systems (RABS). These customized assemblies can include all critical Cytiva components, such as Allegro™ 2D biocontainers serving as surge bags (alternatives to buffer tanks), sterilizing liquid filters, sterilizing gas filters, sterile connectors, Allegro™ single-use filling needles, and various other components including different tubing materials and sizes, connectors, single-use stainless steel needles, rapid transfer port (RTP) bags, small color-coded tubings, or cable ties.
Our final fill assemblies are supplied gamma-irradiated, ready-to-use, out-of-the-box, and are supported by a comprehensive documentation package.
With many years of experience in single-use manufacture, we are your trusted partner to deliver perfectly fit custom solutions at the most critical stage of your process and in close collaboration with your filling machine supplier. Reach out to your sales representative to discuss your needs and explore solutions.