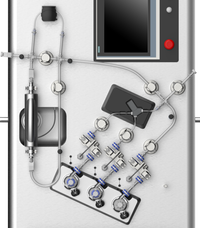
NanoAssemblr™ Blaze nanoparticle formulation system
Process development systems with a single-use cartridge for the formulation of lipid nanoparticles (LNPs)
NanoAssemblr™ Blaze nanoparticle formulation system
Process development systems with a single-use cartridge for the formulation of lipid nanoparticles (LNPs)
Volume
Overview
The NanoAssemblr™ Blaze and Blaze+ systems streamline the process development of lipid nanoparticle (LNP) formulation, enabling the efficient scaling from early research through preclinical and early clinical development. With flexible volume options and a closed system environment, these systems support early chemistry, manufacturing, and control (CMC) studies and large-scale protocol development for upstream and downstream operations, providing valuable insights into processes and consumables required for GMP manufacturing.
- Efficient: Validate end-to-end clinical manufacturing processes, including tangential flow filtration (TFF), sterile filtration, and fill/finish operations on a preclinical system with a simplified setup and shorter run times, saving time and resources.
- Flexible volume options: Select the Blaze system for low-volume applications (up to 1 L) or upgrade to the Blaze+ system for high-volume applications (up to 10 L), enabling toxicity, stability, and large-cohort in vivo animal studies.
- Closed system environment: The Blaze+ system provides a closed system environment that enables external vessel connections and includes a software upgrade, reducing the risk of contamination during scale-up.
- Scalable: Easily transfer critical process parameters (CPPs) to the NanoAssemblr™ commercial formulation system, enabling smooth technology transfer to a cGMP facility.
- Reproducible: NxGen™ technology ensures consistent, controlled mixing conditions, enabling reproducible, robust particle formation.
NxGen™ cartridges and tubing kits
Single-use NxGen™ cartridges reduce the risk of cross-contamination and are available with or without in-line dilution, which automates a key process and streamlines the LNP workflow. The cartridges use the same mixer as the NanoAssemblr™ Ignite+ and NanoAssemblr™ commercial formulation system, enabling the direct transfer of CPPs from bench to process development and clinical and commercial production.
The single-use tubing kit enables external vessel connections and a closed-system environment for the Blaze+ system.
Starting June 2025, customers can purchase the new NxGen™ Blaze cartridges for the NanoAssemblr™ Blaze nanoparticle formulation system and will have the option to reuse them. Blaze system NxGen™ cartridges remain validated for single use only, up to 1 L on the Blaze system or 10 L on the Blaze+ system. Further information can be found in the Blaze user guide.
Learn more
Learn how the NanoAssemblr™ Blaze and Blaze+ systems enable process development in LNP formulation during preclinical development of LNPs.
Consistent physiological responses in vivo using Blaze and Blaze+ to scale up
EPO-encoded mRNA-LNPs increase the hematocrit response consistently in vivo using the NanoAssemblr™ family of systems, including the Blaze system. mRNA-LNPs were formulated at increasing flow rates and scales, followed by downstream processing using ultrafiltration or TFF. C57BL mice were treated with a single intravenous (IV) injection of LNPs, and blood hematocrit levels were assessed 7 days later.
Scalable NxGen™ technology
NxGen™ technology enables controlled and reproducible mixing conditions through its innovative mixing architecture, consisting of unique toroidal structures within the flow route. It is designed to enable the transfer of CPPs during scale-up using the NanoAssemblr™ family of formulation systems.
Maintain critical quality attributes (CQAs) from the NanoAssemblr™ Ignite to Blaze systems and the NanoAssemblr™ commercial formulation system
Self-amplifying RNA (saRNA)-LNPs prepared using NxGen™ technology maintain physicochemical characteristics throughout scale-up to commercial manufacturing. Size, polydispersity index (PDI), and encapsulation efficiency are consistent using A) the NxGen™, NxGen™ 500, and NxGen™ commercial cartridge 48 L/h and B) from Ignite+ or early preclinical development through to the commercial formulation system for clinical and commercial manufacturing. Error bars represent 1 standard deviation, and comparison values are from a post-hoc Tukey test after one-way ANOVA.
Minimize process development and enable the transfer of manufacturing processes to GMP manufacturing
saRNA-LNPs are biologically potent in vitro and in vivo, inducing expression of SARS-CoV-2 antigen and robust immune responses. A) BHK 570 cells were transfected with decreasing amounts of saRNA-LNPs and B) the percentage of cells expressing SARS-CoV-2 spike protein was measured using an anti-spike conjugated AlexaFluor488 antibody with 95% confidence intervals in shaded areas. C) EC50 values were similar across systems. Error bars represent 95% confidence intervals. D) BALB/c mice were used for a 42-day prime and boost dose study. E) Robust SARS-CoV-2-specific IgG responses in serum were observed at day 21 and 42 post-injection for each condition. Error bars are 1 standard deviation. 1x PBS vs instrument comparison p-value for a given time point using post-hoc Tukey test after one-way ANOVA (p≤.05: *, p≤.01: **, p≤.001: ***, p≤.0001: ****).