Summary 1
Research and process development
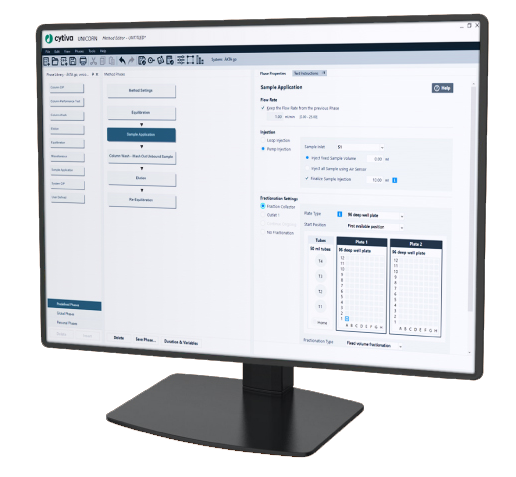
UNICORN™ system control software provides built-in knowledge for planning and controlling runs, as well as analyzing results. The software can control chromatography, rocking bioreactor, filtration, and oligonucleotide synthesis systems. Fully scalable, the UNICORN™ software platform is suitable for use in small-scale research all the way to full-scale GMP manufacturing.
For lab-scale chromatography systems, the Method Editor provides a choice of predefined methods for different techniques and maintenance procedures. Integrated tools (system dependent) are available for design of experiments (DoE) and Column Logbook. For automated buffer preparation, the BufferPro tool supports quick and easy buffer creation.
UNICORN™ control software is activated via e-license for the workstation plus optional components. Add remote and dry licenses or UNICORN™ online capabilities to access the equipment and database from outside the lab space. License packages are available for convenient ordering with new equipment.
Summary 2
GMP manufacturing scale
UNICORN™ control software is based on an integrated controller and an intuitive computer-based interface. The software can control chromatography, rocking bioreactor, filtration, and oligonucleotide synthesis systems. The run sequence is fully determined by the end-user for maximum control of the process. A graphical interface helps you create the process sequence. Advanced users can perform conventional line programming. Interactive process picture and simplified evaluation provide ease of use for all operators.
The software provides robust database handling and user security levels for data security and GAMP 5 support and is compatible with 21 CFR pt 11 requirements for regulatory agencies. An optional extension is available to enable data transfer via OPC HDA into the software. The extension lets you take advantage of the evaluation capabilities in UNICORN™ software.
UNICORN™ control software is activated via e-license for the workstation plus optional components. Add remote and dry licenses to access the equipment and database from outside the GMP space. License packages are available for convenient ordering with new equipment.